BeiwutineCAS# 76918-93-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 76918-93-9 | SDF | Download SDF |
| PubChem ID | 101289637 | Appearance | Powder |
| Formula | C33H45NO12 | M.Wt | 647.7 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
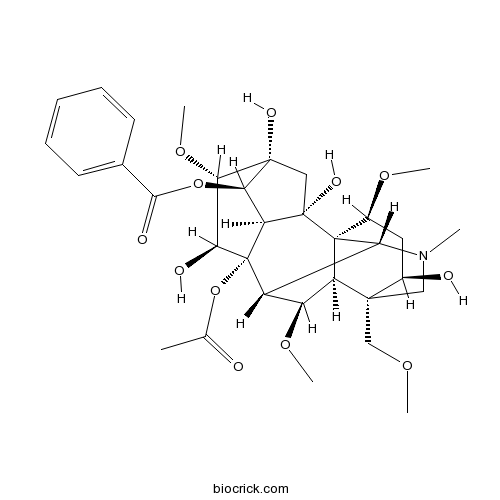
| Chemical Name | [(1R,2S,3S,4R,5R,6S,7S,8S,9R,10R,13R,14R,16S,17R,18R)-8-acetyloxy-2,5,7,14-tetrahydroxy-6,16,18-trimethoxy-13-(methoxymethyl)-11-methyl-11-azahexacyclo[7.7.2.12,5.01,10.03,8.013,17]nonadecan-4-yl] benzoate | ||
| SMILES | CC(=O)OC12C3C(C4C5(CN(C3C4(C(CC5O)OC)C6(C1C(C(C6)(C(C2O)OC)O)OC(=O)C7=CC=CC=C7)O)C)COC)OC | ||
| Standard InChIKey | GPTAWZLFSGYZGC-GWGVWUHLSA-N | ||
| Standard InChI | InChI=1S/C33H45NO12/c1-16(35)46-33-20-21(43-5)22-29(15-41-3)14-34(2)24(20)32(22,19(42-4)12-18(29)36)31(40)13-30(39,27(44-6)25(33)37)26(23(31)33)45-28(38)17-10-8-7-9-11-17/h7-11,18-27,36-37,39-40H,12-15H2,1-6H3/t18-,19+,20+,21+,22-,23+,24-,25+,26-,27+,29+,30-,31+,32-,33+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Beiwutine Dilution Calculator

Beiwutine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5439 mL | 7.7196 mL | 15.4392 mL | 30.8785 mL | 38.5981 mL |
| 5 mM | 0.3088 mL | 1.5439 mL | 3.0878 mL | 6.1757 mL | 7.7196 mL |
| 10 mM | 0.1544 mL | 0.772 mL | 1.5439 mL | 3.0878 mL | 3.8598 mL |
| 50 mM | 0.0309 mL | 0.1544 mL | 0.3088 mL | 0.6176 mL | 0.772 mL |
| 100 mM | 0.0154 mL | 0.0772 mL | 0.1544 mL | 0.3088 mL | 0.386 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Taiwanin C
Catalog No.:BCN9606
CAS No.:14944-34-4
- Stepharine
Catalog No.:BCN9605
CAS No.:2810-21-1
- 7,7'-Dihydrotaiwanin C
Catalog No.:BCN9604
CAS No.:216955-79-2
- Menisdaurilide
Catalog No.:BCN9603
CAS No.:67765-59-7
- Aquilegiolide
Catalog No.:BCN9602
CAS No.:94481-79-5
- 16-Oxoserratenediol
Catalog No.:BCN9601
CAS No.:24513-52-8
- Norushinsunine
Catalog No.:BCN9600
CAS No.:3175-84-6
- 3β-Hydroxy-7β,25-dimethoxycucurbita-5,23-dien-19-al
Catalog No.:BCN9599
CAS No.:85372-69-6
- (2R,3R)-Glucodistylin
Catalog No.:BCN9598
CAS No.:27297-45-6
- Sinococuline
Catalog No.:BCN9597
CAS No.:109351-36-2
- Tannagine
Catalog No.:BCN9596
CAS No.:123750-34-5
- Junosine
Catalog No.:BCN9595
CAS No.:103956-34-9
- Apigenin 7-O-(2'',6''-di-O-E-p-coumaroyl)glucoside
Catalog No.:BCN9608
CAS No.:1448779-19-8
- Karavilagenin F
Catalog No.:BCN9609
CAS No.:1639024-15-9
- 3-O-(p-Hydroxybenzoyl)serratriol
Catalog No.:BCN9610
CAS No.:1448534-93-7
- 5-Methoxyjusticidin A
Catalog No.:BCN9611
CAS No.:205505-62-0
- Taiwanin E
Catalog No.:BCN9612
CAS No.:22743-05-1
- 6,7-Di-O-acetylsinococuline
Catalog No.:BCN9613
CAS No.:1054312-81-0
- Illiciumlignan D
Catalog No.:BCN9614
CAS No.:2237239-36-8
- Tuberculatin
Catalog No.:BCN9615
CAS No.:90706-10-8
- Greveichromenol
Catalog No.:BCN9616
CAS No.:35930-29-1
- Cernuine
Catalog No.:BCN9617
CAS No.:6880-84-8
- Karavilagenin B
Catalog No.:BCN9618
CAS No.:912329-02-3
- Heteropeucenin 7-methyl ether
Catalog No.:BCN9619
CAS No.:26213-95-6
[Content determination of four diester diterpenoid alkaloids in leaves of Aconitum kusnezoffii by HPLC].[Pubmed:29600653]
Zhongguo Zhong Yao Za Zhi. 2018 Feb;43(4):766-771.
This present study is to develop an HPLC method for simultaneous determination of four diester diterpenoid alkaloids, Beiwutine, mesaconitine, hypaconitine and aconitine in the leaves of Aconitum kusnezoffii, so as to provide evidence of the quality control of this herb. The four constituents were measured on a Waters XBridge CC(1)(8) column(4.6 mmchi250 mm, 5 mum). The mobile phase was acetonitrile-40 mmol.L(-)(1) ammonium acetate solution(adjusted pH to 10.5 with ammonia solution)(33:67) with isocratic elution at a flow rate of 1.0 mL.min(-)(1); the detection wavelength was 235 nm; the column temperature was 30 degrees C, and the injection volume was 10 muL. Next, this contents of the four diester diterpenoid alkaloids in 12 samples were 0.025 5-0.088 5, 0.039 1-0.071 5, 0.026 6-0.081 0 and 0.008 12-0.031 2 mg.g(-)(1), respectively. Next, this method has been successfully applied to the analysis of A. kusnezoffii folium in different harvest periods. The contents of the four alkaloids decreased primarily, and then increased with the postponing of harvest. The established method is proved to be accurate and sensitive for the determination of alkaloids in A. kusnezoffii folium, and may be useful for the quality improvement of this herbal medicine. Moreover, these results indicated the scientific significance for the toxicity and the suitable harvest time of this herb.
[Isolation and structure identification of C19 diterpenoid alkaloids from Aconitum carmichaeli by high-speed counter-current chromatography].[Pubmed:21847978]
Se Pu. 2011 May;29(5):430-4.
Three C19 diterpenoid alkaloids were isolated and purified from the lateral roots of Aconitum carmichaeli Debx. (Fuzi in Chinese) by high-speed counter-current chromatography (HSCCC). A mixture of n-hexane-ethyl acetate-methanol-water (3:5:4:5, v/v/v/v) was used as the two phase solvent system. The lower phase was used as the mobile phase and was operated at a flow rate of 2.0 mL/min, while the apparatus was rotated at 850 r/min, and the detection wavelength was at 235 nm. Under these conditions, 15.3 mg of Beiwutine, 35.1 mg of mesaconitine and 22.7 mg of hypaconitine were obtained from 90 mg of crude extract in one-step separation with the purities of 97.9%, 96.2% and 99.2%, respectively, determined by high performance liquid chromatography. The structures of these three compounds were identified by electrospray ionization mass spectrometry (ESI-MS), 1H-nuclear magnetic resonance (1H-NMR) and 13C-NMR. The results indicate that HSCCC is a powerful technique for the purification of diterpenoid alkaloids from the lateral roots of Aconitum carmichaeli Debx.
[Determination of alkaloids in Radix Aconiti Lateralis Preparata by RP-ion-pair HPLC].[Pubmed:16856485]
Yao Xue Xue Bao. 2006 Apr;41(4):365-9.
AIM: To separate and quantitatively determine six alkaloids: aconitine, mesaconitine, hypaconitine, Beiwutine, benzoylaconine and benzoylmesaconine in the Chinese traditional medicine Radix Aconiti Lateralis Preparata (Fuzi). METHODS: A RP-ion-pair HPLC method was established. An AichromBond-1 C18 column was used at a column-temperature of 35 degrees C. The mobile phase was CH3CN5 mmol x L(-1) NaH2PO4(50:50) containing 7 mmol x L(-1) SDS at a flow-rate of 1.0 mL x min(-1). The detector was set at UV 235 nm. RESULTS: These six alkaloids can be completely separated and determined quantitatively. CONCLUSION: This method is accurate and suitable for the determination of six alkaloids in Fuzi.
Norditerpenoid alkaloids from Aconitum manshuricum.[Pubmed:29435883]
J Nat Med. 2006 Jul;60(3):255-257.
A new norditerpenoid alkaloid, manshuritine (1) has been isolated from Aconitum manshuricum, together with seven known alkaloids; beiwudine (2), Beiwutine (3), 16-epi-pyromesaconitine (4), mesaconitine (5), aconitine (6), hypaconitine (7) and 14-benzoylmesaconine (8). The structure of the new compound was elucidated on the basis of spectral data and chemical correlations.
A QSAR analysis of toxicity of Aconitum alkaloids.[Pubmed:15548242]
Fundam Clin Pharmacol. 2004 Dec;18(6):699-704.
Biological activity of Aconitum alkaloids may be related to their toxicity rather than to a specific pharmacological action. A Quantitative structure-activity relationships (QSAR) analysis was performed on the following two groups of alkaloids: compounds with an aroyl/aroyloxy group at R(14) position (yunaconitine, bulleyaconitine, aconitine, Beiwutine, nagarine, 3-acetyl aconitine, and penduline), and compounds with the aroyloxy group at R(4) position (N-deacetyllappaconitine, lappaconitine, ranaconitine, N-deacetylfinaconitine, N-deacetylranaconitine). The LD(50) (micromol/kg) of the 12 alkaloids were obtained from the literature. LD(50) was significantly lower in group 1 than in group 2. The steric and core-core repulsion energies were significantly higher in group 1. The total energy and heat of formation and electronic energies were significantly lower in group 1. The reactivity index of N, C1', C4' and C6' were similar between groups. The reactivity index of C2' was significantly higher and the reactivity index of C3' and C5' were significantly lower in group 1. Log P and pKa were similar between groups. Molecular weight was significantly higher in group 1. A significant linear relationship was observed between log LD(50) and either analgesic log ED(50) or local anesthetic log ED(50). The LD(50)/analgesic ED(50) obtained from average values was 5.9 for group 1 and 5.0 for group 2. However, the LD(50)/local anesthetic ED(50) was 40.4 and 318, respectively. The study supports that the analgesic effects of these alkaloids are secondary to their toxic effects whereas alkaloids from group 2 are susceptible to be further studied as local anesthetic agents.
[Effects of guan-fu base a on experimental cardiac arrhythmias and myocardial contractility].[Pubmed:8571777]
Yao Xue Xue Bao. 1995;30(8):577-82.
Guan-fu base A (GFA) is a terpenoid alkaloid isolated from the tuber of Aconitum coreanum in our institute. GFA (20-30 mg.L-1) reduced the ventricular tachycardia (VT) and ventricular fibrillation (VF) rate induced by K(+)-free and high Ca2+ solution in Langendorf heart of rats. Pretreatment of conscious rats with GFA 2.5-10 mg.kg-1 iv, increased the amount of Beiwutine necessary to produce arrhythmias. Ouabain-induced VT in conscious dogs was reverted to sinus rhythm in 1-2 min by iv GFA 9-10 mg.kg-1. GFA 10-20 mg.kg-1 iv was also found to be effective in protecting anesthetized dogs from atrial fibrillation induced by topical application of ACh. GFA 10 mg.kg-1 iv obviously decreased heart rate and prolonged the P-R interval, but slightly affected the myocardial contractility in anesthetized dogs. GFA showed no obvious effect on stroke volume and cardiac output in conscious dogs. In conclusion, GFA showed therapeutic and prophylactic effect on different models of experimental arrhythmias without causing marked effect on myocardial contractility.


