NorushinsunineCAS# 3175-84-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 3175-84-6 | SDF | Download SDF |
| PubChem ID | 10378981 | Appearance | Brown powder |
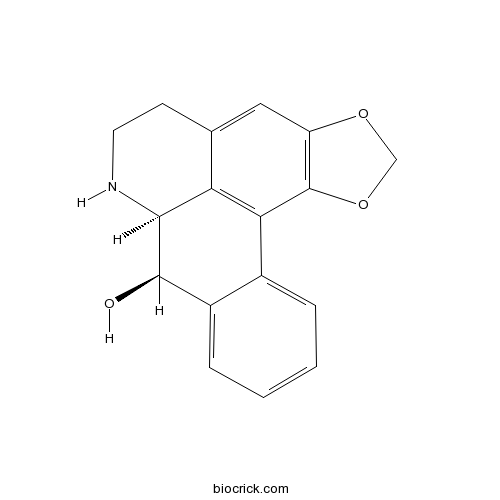
| Formula | C17H15NO3 | M.Wt | 281.3 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (12S,13R)-3,5-dioxa-11-azapentacyclo[10.7.1.02,6.08,20.014,19]icosa-1(20),2(6),7,14,16,18-hexaen-13-ol | ||
| SMILES | C1CNC2C(C3=CC=CC=C3C4=C2C1=CC5=C4OCO5)O | ||
| Standard InChIKey | CKIYSMRPIBQTHQ-JKSUJKDBSA-N | ||
| Standard InChI | InChI=1S/C17H15NO3/c19-16-11-4-2-1-3-10(11)14-13-9(5-6-18-15(13)16)7-12-17(14)21-8-20-12/h1-4,7,15-16,18-19H,5-6,8H2/t15-,16+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Norushinsunine Dilution Calculator

Norushinsunine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5549 mL | 17.7746 mL | 35.5492 mL | 71.0985 mL | 88.8731 mL |
| 5 mM | 0.711 mL | 3.5549 mL | 7.1098 mL | 14.2197 mL | 17.7746 mL |
| 10 mM | 0.3555 mL | 1.7775 mL | 3.5549 mL | 7.1098 mL | 8.8873 mL |
| 50 mM | 0.0711 mL | 0.3555 mL | 0.711 mL | 1.422 mL | 1.7775 mL |
| 100 mM | 0.0355 mL | 0.1777 mL | 0.3555 mL | 0.711 mL | 0.8887 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3β-Hydroxy-7β,25-dimethoxycucurbita-5,23-dien-19-al
Catalog No.:BCN9599
CAS No.:85372-69-6
- (2R,3R)-Glucodistylin
Catalog No.:BCN9598
CAS No.:27297-45-6
- Sinococuline
Catalog No.:BCN9597
CAS No.:109351-36-2
- Tannagine
Catalog No.:BCN9596
CAS No.:123750-34-5
- Junosine
Catalog No.:BCN9595
CAS No.:103956-34-9
- Citrusinine I
Catalog No.:BCN9594
CAS No.:86680-32-2
- 6a,7-Dehydroboldine
Catalog No.:BCN9593
CAS No.:91599-23-4
- Boschnaloside
Catalog No.:BCN9592
CAS No.:72963-55-4
- Taccaoside E
Catalog No.:BCN9591
CAS No.:1858199-00-4
- 3'-Hydroxy-3,5,8,4',5'-pentamethoxy-6,7-methylenedioxyflavone
Catalog No.:BCN9590
CAS No.:82668-94-8
- 11β,13-Dihydrotaraxinic acid
Catalog No.:BCN9589
CAS No.:1274668-83-5
- Isostephodeline
Catalog No.:BCN9588
CAS No.:56648-85-2
- 16-Oxoserratenediol
Catalog No.:BCN9601
CAS No.:24513-52-8
- Aquilegiolide
Catalog No.:BCN9602
CAS No.:94481-79-5
- Menisdaurilide
Catalog No.:BCN9603
CAS No.:67765-59-7
- 7,7'-Dihydrotaiwanin C
Catalog No.:BCN9604
CAS No.:216955-79-2
- Stepharine
Catalog No.:BCN9605
CAS No.:2810-21-1
- Taiwanin C
Catalog No.:BCN9606
CAS No.:14944-34-4
- Beiwutine
Catalog No.:BCN9607
CAS No.:76918-93-9
- Apigenin 7-O-(2'',6''-di-O-E-p-coumaroyl)glucoside
Catalog No.:BCN9608
CAS No.:1448779-19-8
- Karavilagenin F
Catalog No.:BCN9609
CAS No.:1639024-15-9
- 3-O-(p-Hydroxybenzoyl)serratriol
Catalog No.:BCN9610
CAS No.:1448534-93-7
- 5-Methoxyjusticidin A
Catalog No.:BCN9611
CAS No.:205505-62-0
- Taiwanin E
Catalog No.:BCN9612
CAS No.:22743-05-1
Isoquinoline alkaloids from Asimina triloba.[Pubmed:30453785]
Nat Prod Res. 2019 Oct;33(19):2823-2829.
A new aporphine glycoside, (-)-anolobine-9-O-beta-D-glucopyranoside was isolated from the twigs of pawpaw (Asimina triloba) along with 7 known alkaloids including five aporphine alkaloids (anolobine, nornuciferine, Norushinsunine, liriodenine, and lysicamine), a proaporhine alkaloid (stepharine), and a tetrahydrobenzylisoquinoline alkaloid (coclaurine). Among these compounds, nornuciferine, lysicamine, stepharine, and coclaurine are reported for the first time from this plant. The structure of the new compound was elucidated by spectroscopic methods, including 1 D, 2 D NMR, and HRESI-MS. The absolute configuration of compounds 1, 2, 7 and 8 was determined by CD experiment.
Aporphine alkaloids and cytotoxic lignans from the roots of Illigera luzonensis.[Pubmed:21315382]
Phytochemistry. 2011 Apr;72(6):523-32.
Six aporphine alkaloids, (+)-(S)-N-butyrylcaaverine (1), (+)-(S)-N-propionylcaaverine (2), (+)-(S)-N-acetylcaaverine (3), (+)-(6aR,7R)-N-butyrylNorushinsunine (4), (+)-(6aR,7R,E)-N-(but-2-enoyl)Norushinsunine (5), and N-formyldehydrocaaverine (6) were isolated from the roots of Illigera luzonensis, together with 16 known compounds. Their structures were determined through spectroscopic and MS analyses. Among the isolates, (-)-deoxypodophyllotoxin (13) was the most cytotoxic, with IC(50) values of 0.0057, 0.0067, 0.00004, and 0.0035mug/mL, respectively, against DLD-1, CCRF-CEM, HL-60, and IMR-32 cell lines. In addition, (-)-yatein (12) exhibited cytotoxic effects, with IC(50) values of 0.81, 0.20, and 0.59mug/mL, respectively, against DLD-1, CCRF-CEM, and HL-60 cell lines.
Antiplasmodial activity of aporphine alkaloids and sesquiterpene lactones from Liriodendron tulipifera L.[Pubmed:20826204]
J Ethnopharmacol. 2011 Jan 7;133(1):26-30.
AIM OF THE STUDY: The objective of this study was to isolate and characterize the active constituents of the traditionally used antimalarial plant Liriodendron tulipifera by antiplasmodial-assay guided fractionation. MATERIALS AND METHODS: Bark and leaves were extracted with solvents of increasing polarity. Fractions were generated using flash chromatography, counter current chromatography and preparative HPLC and subjected to in vitro antiplasmodial and cytotoxicity assays. Active fractions were subjected to further fractionation until pure compounds were isolated, for which the IC(50) values were calculated. RESULTS AND DISCUSSION: Six known aporphine alkaloids, asimilobine (1), Norushinsunine (2), norglaucine (3), liriodenine (4), anonaine (5) and oxoglaucine (6) were found to be responsible for the antiplasmodial activity of the bark. Leaves yielded two known sesquiterpene lactones, peroxyferolide (7) and lipiferolide (8) with antiplasmodial activity. The antiplasmodial activity of (2) (IC(50)=29.6 mug/mL), (3) (IC(50)=22.0 mug/mL), (6) (IC(50)=9.1 mug/mL), (7) (IC(50)=6.2 mug/mL) and (8) (IC(50)=1.8 mug/mL) are reported for the first time. CONCLUSION: This work supports the historical use of Liriodendron tulipifera as an antimalarial remedy of the United States and characterizes its antiplasmodial constituents.
Vasodilator effects of liriodenine and norushinsunine, two aporphine alkaloids isolated from Annona cherimolia, in rat aorta.[Pubmed:7568337]
Pharmacology. 1995 Jun;50(6):380-7.
The effect of two aporphines, liriodenine and Norushinsunine, isolated from Annona cherimolia, were studied in the rat aorta in order to examine their mechanism of action. Both alkaloids (10(-7) - 10(-4) mol/l) showed relaxant effects on the contractions elicited by 10(-6) mol/l noradrenaline (NA) or 80 mmol/1 KCl, but, while liriodenine showed a nonspecific relaxant action on both spasmogens, Norushinsunine was more potent on KCl-induced contraction. In Ca2+ free medium, both alkaloids (0.1 mmol/l) inhibited the responses elicited by NA, but not those elicited by caffeine. This inhibitory action occurred when the alkaloids were present during the release of the Ca2+ internal stores or during the refilling process. These results suggest that the two aporphines show a relaxant action in rat aorta which is mediated by an interaction with alpha1-adrenoceptors and an alteration of the Ca2+ entry via voltage-operated channels. Norushinsunine exhibits a certain degree of selectivity as an L-type Ca2+ channel blocker.


