Taiwanin CCAS# 14944-34-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 14944-34-4 | SDF | Download SDF |
| PubChem ID | 363127 | Appearance | Powder |
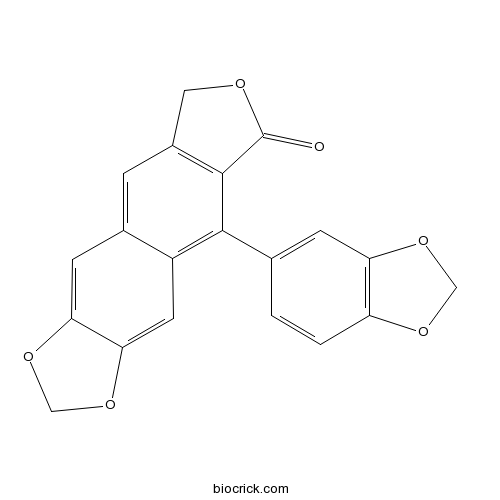
| Formula | C20H12O6 | M.Wt | 348.3 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-(1,3-benzodioxol-5-yl)-8H-[2]benzofuro[5,6-f][1,3]benzodioxol-6-one | ||
| SMILES | C1C2=C(C(=C3C=C4C(=CC3=C2)OCO4)C5=CC6=C(C=C5)OCO6)C(=O)O1 | ||
| Standard InChIKey | YMGOOHXUOWZQOE-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H12O6/c21-20-19-12(7-22-20)3-11-5-16-17(26-9-25-16)6-13(11)18(19)10-1-2-14-15(4-10)24-8-23-14/h1-6H,7-9H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Taiwanin C Dilution Calculator

Taiwanin C Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8711 mL | 14.3554 mL | 28.7109 mL | 57.4218 mL | 71.7772 mL |
| 5 mM | 0.5742 mL | 2.8711 mL | 5.7422 mL | 11.4844 mL | 14.3554 mL |
| 10 mM | 0.2871 mL | 1.4355 mL | 2.8711 mL | 5.7422 mL | 7.1777 mL |
| 50 mM | 0.0574 mL | 0.2871 mL | 0.5742 mL | 1.1484 mL | 1.4355 mL |
| 100 mM | 0.0287 mL | 0.1436 mL | 0.2871 mL | 0.5742 mL | 0.7178 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Stepharine
Catalog No.:BCN9605
CAS No.:2810-21-1
- 7,7'-Dihydrotaiwanin C
Catalog No.:BCN9604
CAS No.:216955-79-2
- Menisdaurilide
Catalog No.:BCN9603
CAS No.:67765-59-7
- Aquilegiolide
Catalog No.:BCN9602
CAS No.:94481-79-5
- 16-Oxoserratenediol
Catalog No.:BCN9601
CAS No.:24513-52-8
- Norushinsunine
Catalog No.:BCN9600
CAS No.:3175-84-6
- 3β-Hydroxy-7β,25-dimethoxycucurbita-5,23-dien-19-al
Catalog No.:BCN9599
CAS No.:85372-69-6
- (2R,3R)-Glucodistylin
Catalog No.:BCN9598
CAS No.:27297-45-6
- Sinococuline
Catalog No.:BCN9597
CAS No.:109351-36-2
- Tannagine
Catalog No.:BCN9596
CAS No.:123750-34-5
- Junosine
Catalog No.:BCN9595
CAS No.:103956-34-9
- Citrusinine I
Catalog No.:BCN9594
CAS No.:86680-32-2
- Beiwutine
Catalog No.:BCN9607
CAS No.:76918-93-9
- Apigenin 7-O-(2'',6''-di-O-E-p-coumaroyl)glucoside
Catalog No.:BCN9608
CAS No.:1448779-19-8
- Karavilagenin F
Catalog No.:BCN9609
CAS No.:1639024-15-9
- 3-O-(p-Hydroxybenzoyl)serratriol
Catalog No.:BCN9610
CAS No.:1448534-93-7
- 5-Methoxyjusticidin A
Catalog No.:BCN9611
CAS No.:205505-62-0
- Taiwanin E
Catalog No.:BCN9612
CAS No.:22743-05-1
- 6,7-Di-O-acetylsinococuline
Catalog No.:BCN9613
CAS No.:1054312-81-0
- Illiciumlignan D
Catalog No.:BCN9614
CAS No.:2237239-36-8
- Tuberculatin
Catalog No.:BCN9615
CAS No.:90706-10-8
- Greveichromenol
Catalog No.:BCN9616
CAS No.:35930-29-1
- Cernuine
Catalog No.:BCN9617
CAS No.:6880-84-8
- Karavilagenin B
Catalog No.:BCN9618
CAS No.:912329-02-3
Chemical Constituents and an Antineuroinflammatory Lignan, Savinin from the Roots of Acanthopanax henryi.[Pubmed:30915141]
Evid Based Complement Alternat Med. 2019 Feb 21;2019:1856294.
The phytochemical investigation on the roots of Acanthopanax henryi (Araliaceae) resulted in the discovery of twenty compounds whose chemical structures were elucidated by the analysis of 1D-, 2D-NMR, mass spectrometry data, other physicochemical properties, and a comparison of the spectral data with the literature. They were identified as (-)-sesamin (1), helioxanthin (2), savinin (3), Taiwanin C (4), 6-methoxy-7-hydroxycoumarin (5), behenic acid (6), 3-O-caffeoyl-quinic acid (7), 5-O-caffeoyl-quinic acid (8), 1,3-di-O-caffeoyl-quinic acid (9), 1,4-di-O-caffeoyl-quinic acid (10), 1,5-di-O-caffeoyl-quinic acid (11), (+)-threo-(7R,8R)-guaiacylglycerol-beta-coniferyl aldehyde ether (12), (+)-erythro-(7S,8R)-guaiacylglycerol-beta-coniferyl aldehyde ether (13), ferulic acid (14), caffeic acid (15), stigmasterol (16), beta-sitosterol (17), adenosine (18), syringin (19), and trans-coniferin (20). Among these isolates, compound 3 showed inhibitory activity against lipopolysaccharide- (LPS-) induced nitric oxide (NO) and prostaglandin E2 (PGE2) production with IC50 values of 2.22 +/- 0.11 and 2.28 +/- 0.23 muM, respectively. The effects of compound 3 were associated with the suppression of LPS-induced expression of the inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) protein. Furthermore, compound 3 negatively regulated the production of interleukin- (IL-) 1beta and tumor-necrosis factor- (TNF-) alpha at the transcriptional level in LPS-stimulated BV2 microglial cells. These antineuroinflammatory effects of compound 3 were mediated by p38 mitogen-activated protein kinase (MAPK).
Taiwanin C elicits apoptosis in arecoline and 4-nitroquinoline-1-oxide-induced oral squamous cell carcinoma cells and hinders proliferation via epidermal growth factor receptor/PI3K suppression.[Pubmed:30884126]
Environ Toxicol. 2019 Jun;34(6):760-767.
Oral Squamous Cell Carcinoma (OSSC) is a major life-threatening disease with high incidence in the Southeast Asian countries. Chronic exposure to arecoline causes genetic changes in the epithelial cells of the oral mucosa, induces proliferation through activation of the EGF receptor and promotes downstream COX-2 expression. Taiwanin C, a podophyllotoxin derived from Taiwania cryptomerioides Hayata is known to inhibit COX activity and to hinder PGE2 production in macrophages. In this study a tumor cell line T28 and a non-tumor cell line N28 derived from mice OSCC models were used to study the effect of Taiwanin C on PGE2 associated COX-2 expression and cell cycle regulators. Taiwanin C activated p21 protein expression, down-regulated cell cycle regulatory proteins, elevated apoptosis and down-regulated p-PI3K/p-Akt survival mechanism in T28 oral cancer cells. Our results therefore emphasize the therapeutic potential of Taiwanin C against arecoline-induced oral cancer.
Down-regulation of beta-catenin and the associated migration ability by Taiwanin C in arecoline and 4-NQO-induced oral cancer cells via GSK-3beta activation.[Pubmed:27648737]
Mol Carcinog. 2017 Mar;56(3):1055-1067.
Cancer is one of the leading causes of death worldwide, and oral squamous cell carcinoma (OSCC) accounts for almost a sixth of all reported cancers. Arecoline, from areca nut is known to enhance carcinogenesis in oral squamous cells. The objective of this study is to determine the effect of Taiwanin C, from Taiwania cryptomerioides Hayata against Arecoline-associated carcinogenesis. An OSCC model was created in C57BL/6J Narl mice by administrating 0.5 mg mL(-1) arecoline with 0.2 mg mL(-1) 4-NQO carcinogen for 8 and 28 wk to mimic the etiology of oral cancer patients in Asia. Mice were sacrificed and two cell lines, T28 from the tumor and N28 cancerous cell line from the surrounding non tumor area, were established. Taiwanin C showed effective anti-tumor activity in nude mice models. Taiwanin C significantly inhibited the cell viability of T28 cells in a dose dependent manner, but did not inflict any effect on N28 normal cells. Taiwanin C treatment inhibited the migration ability of T28 cells in a dose dependent manner as determined by wound healing and migration assays. Taiwanin C also reduced the levels of beta-catenin and its downstream metastatic proteins, Tbx3 and c-Myc. Besides, Taiwanin C inhibited the nuclear accumulation of beta-catenin and induced beta-catenin degradation via proteasome-mediated pathway. Moreover, Taiwanin C enhanced GSK-3beta and reduced the p-ser(9) GSK-3beta protein level to inactivate Wnt signaling. Taken together, Taiwanin C blocked the cell migration effects of T28 cells mediated through the activation of GSK-3beta to enhance protein degradation and reduce nuclear accumulation of beta-catenin. (c) 2016 Wiley Periodicals, Inc.
Taiwanin C selectively inhibits arecoline and 4-NQO-induced oral cancer cell proliferation via ERK1/2 inactivation.[Pubmed:26537528]
Environ Toxicol. 2017 Jan;32(1):62-69.
Arecoline, the most abundant alkaloid in betel nut is known to promote abnormal proliferation of epithelial cells by enhancing epidermal growth factor receptor (EGFR) activation and cyclooxygenase-2 (COX2) expression. Taiwanin C, a naturally occurring lignan extracted from Taiwania cryptomerioides, has been found to be a potential inhibitor of COX2 expression. Based on the MTT assay results, Taiwanin C was found to be effective in inhibiting the tumorous T28 cell than the non-tumorous N28 cells. The modulations in the expression of relevant proteins were determined to understand the mechanism induced by Taiwanin C to inhibit T28 cell proliferation. The levels of activated EGFR and COX2 were found to be abnormally high in the T28 oral cancer cells. However, Taiwanin C was found to inhibit the activation of EGFR and regulated other related downstream proteins and thereby inhibited the T28 cell proliferation. In conclusion the results indicate that Taiwanin C suppresses COX2-EGFR and enhances P27 pathways to suppress arecoline induced oral cancer cell proliferation via ERK1/2 inactivation. (c) 2015 Wiley Periodicals, Inc. Environ Toxicol 32: 62-69, 2017.
Intramolecular dehydro-Diels-Alder reaction affords selective entry to arylnaphthalene or aryldihydronaphthalene lignans.[Pubmed:25061845]
Org Lett. 2014 Aug 15;16(16):4158-61.
Intramolecular dehydro-Diels-Alder (DDA) reactions are performed affording arylnaphthalene or aryldihydronaphthalene lactones selectively as determined by choice of reaction solvent. This constitutes the first report of an entirely selective formation of arylnaphthalene lactones utilizing DDA reactions of styrene-ynes. The synthetic utility of the DDA reaction is demonstrated by the synthesis of Taiwanin C, retrohelioxanthin, justicidin B, isojusticidin B, and their dihydronaphthalene derivatives. Computational methods for chemical shift assignment are presented that allow for regioisomeric lignans to be distinguished.
Synthesis of arylnaphthalene lignan scaffold by gold-catalyzed intramolecular sequential electrophilic addition and benzannulation.[Pubmed:22029795]
J Org Chem. 2011 Dec 16;76(24):9919-33.
An intramolecular approach to generate compounds containing an arylnaphthalene lignan scaffold in high yields is presented. It involves a sequential intramolecular electrophilic attack of carbonyl on arylalkyne followed by benzannulation catalyzed by gold salt. AuCl(3) in combination with AgSbF(6) works better to effect this transformation. Selected products have been converted into arylnaphthalene lactone natural products such as justicidin E, Taiwanin C, and retrojusticidin B.
Chromatographic fingerprint analysis and simultaneous determination of eight lignans in Justicia procumbens and its compound preparation by HPLC-DAD.[Pubmed:21328534]
J Sep Sci. 2011 Mar;34(6):667-74.
HPLC fingerprints were developed for the quality evaluation of Justicia procumbens and its compound preparation, Jian-er syrup, together with the simultaneous quantification of eight arylnaphthalide lignans (6'-hydroxy justicidin B, 6'-hydroxy justicidin A, 6'-hydroxy justicidin C, justicidin B, chinensinaphthol methyl ether, justicidin C, Taiwanin C, and neojusticin A). Samples were separated with a Shiseido Capcell Pak C(18) reversed-phase column (250x4.6 mm id, 5 mum) using acetonitrile and water as the mobile phase. The column temperature was maintained at 35 degrees C and the wavelength of detector was set at 256 nm. For fingerprint analysis, 17 peaks were selected as the characteristic peaks for the evaluation of the similarities among different J. procumbens samples collected in different places. The structures of lignans were confirmed by diagnostic fragments in the positive ESI-MS(n) . The new method was successfully applied for the chromatographic fingerprint analysis and simultaneous determination of eight lignans in its compound preparation, Jian-er syrup. All the results indicated that HPLC fingerprint assay in combination with multi-marker determination afforded a useful method for the quality control of J. procumbens and its compound preparation, Jian-er syrup.
Silver-catalyzed one-pot synthesis of arylnaphthalene lactone natural products.[Pubmed:20450171]
J Nat Prod. 2010 May 28;73(5):811-3.
Naturally occurring arylnaphthalene lactone lignans have demonstrated a variety of valuable medicinal chemistry properties and have therefore been of continued interest to drug discovery research. Our group has demonstrated a silver-catalyzed one-pot synthesis of the arylnaphthalene lactone core using carbon dioxide, phenylpropargyl chloride, and phenylacetylene. This new approach has been employed in the synthesis of six arylnaphthalene lactone natural products: retrochinensin (1), justicidin B (2), retrojusticidin B (3), chinensin (4), justicidin E (5), and Taiwanin C (6). Additionally, an arylnaphthalene lactone regioisomer was isolated (9), which we refer to as isoretrojusticidin B.
Lead compounds for anti-inflammatory drugs isolated from the plants of the traditional oriental medicine in Korea.[Pubmed:18782027]
Inflamm Allergy Drug Targets. 2008 Sep;7(3):195-202.
Effects of compounds isolated from medicinal plants in Korea on prostaglandin E2 (PGE2) production in rat peritoneal macrophages were examined, and mechanism of action of the active constituents was analyzed. The active constituents were as follows; tectorigenin and tectoridin isolated from the rhizomes of Belamcanda chinensis, platycodin D isolated from the roots of Platycodon grandiflorum, imperatorin isolated from the roots of Angelica dahurica, and hyperin isolated from the roots of Acanthopanax chiisanensis. These compounds inhibit the induction of cyclooxygenase-2 (COX-2), thus inhibiting PGE2 production. The chemically synthesized chalcone derivative, 2'-hydroxy-4'-methoxychalcone, also inhibits PGE2 production by suppressing COX-2 induction. In contrast, Taiwanin C isolated from the roots of Acanthopanax chiisanensis inhibited PGE2 production by direct inhibition of COX-1 and COX-2.
Complete assignments of (1)H and (13)C NMR spectral data for arylnaphthalene lignan lactones.[Pubmed:17729238]
Magn Reson Chem. 2007 Oct;45(10):902-4.
In this work we describe the complete (1)H and (13)C NMR analyses of three arylnaphtalene lignan lactones (Taiwanin C, 4-methyl dehydroretrodendrin and justicidin B) using modern NMR techniques such as gCOSY, nonedited gHSQC, gHMBC and NOE experiments. Complete assignment and homonuclear hydrogen coupling constant measurements were performed.
Complete assignments of 1H and 13C NMR data for seven arylnaphthalide lignans from Justicia procumbens.[Pubmed:16625672]
Magn Reson Chem. 2006 Jul;44(7):727-30.
Three new arylnaphthalide lignans named 6'-hydroxy justicidin A (1), 6'-hydroxy justicidin B (2) and 6'-hydroxy justicidin C (3) have been isolated from the whole plant of Justicia procumbens, together with four known ones, neojusticin A (4), chinensinaphthol methyl ester (5), isodiphyllin (6) and Taiwanin C (7). The complete assignments of 1H and 13C NMR chemical shifts for the new lignans and the 13C NMR chemical shifts for the known lignans were obtained for the first time by means of 2D NMR techniques, including HSQC and HMBC.
Lignans from Acanthopanax chiisanensis having an inhibitory activity on prostaglandin E2 production.[Pubmed:15852488]
Phytother Res. 2005 Feb;19(2):103-6.
The chloroform and the ethyl acetate fractions from the roots of Acanthopanax chiisanensis exhibited the significant inhibition of TPA-induced prostaglandin E(2) (PGE(2)) production in rat peritoneal macrophages. Five lignans were isolated from the chloroform fraction and their structures were elucidated as l-sesamin, helioxanthin, savinin, Taiwanin C, and 3-(3,4-dimethoxybenzyl)-2-(3,4-methylenedioxybenzyl)butyrolactone. Among the lignans tested, Taiwanin C showed the most potent inhibitory activity (IC(50) = 0.12 microM) on PGE(2) production with the relative order of potency, Taiwanin C >> 3-(3,4-dimethoxybenzyl)-2-(3,4-methylenedioxybenzyl)butyrolactone > savinin = helioxanthin. l-Sesamin showed no inhibitory activity at 30 microM.
Anti-estrogenic activity of lignans from Acanthopanax chiisanensis root.[Pubmed:15789749]
Arch Pharm Res. 2005 Feb;28(2):186-9.
Anti-estrogenic activity of (-)-sesamin (1), helioxanthin (2), savinin (3), Taiwanin C (4), and 3-(3,4-dimethoxybenzyl)-2-(3,4-methylenedioxybenzyl)butyrolactone (5) isolated from the root of Acanthopanax chiisanensis was tested using Ishikawa cells. Among them, compound 3 exhibited anti-estrogenic activity (IC50 = 4.86 microM).
Inhibition of prostaglandin E(2) production by taiwanin C isolated from the root of Acanthopanax chiisanensis and the mechanism of action.[Pubmed:12392817]
Biochem Pharmacol. 2002 Nov 1;64(9):1345-54.
Five lignans, l-sesamin, savinin, helioxanthin, Taiwanin C, and cis-dibenzylbutyrolactone, were isolated from the root of Acanthopanax chiisanensis (Araliaceae), a Korean medicinal plant, and their inhibitory effects on the production of prostaglandin (PG) E(2) stimulated by 12-O-tetradecanoylphorbol 13-acetate (TPA) in rat peritoneal macrophages were examined. Among the five lignans, Taiwanin C was the most potent (IC(50)=0.12 microM), followed by helioxanthin, cis-dibenzylbutyrolactone, and savinin. l-Sesamin had no effect. Taiwanin C showed no inhibitory effect on the TPA-induced release of radioactivity from [3H]arachidonic acid-labeled macrophages, nor did it inhibit the expression of cyclooxygenase (COX)-2 protein induced by TPA. However, the activities of isolated COX-1 and COX-2 were inhibited by Taiwanin C (IC(50)=1.06 and 9.31 microM, respectively), reflecting the inhibition of both COX-1- and COX-2-dependent PGE(2) production in the cell culture system. These findings suggest that the mechanism of action of Taiwanin C in the inhibition of PGE(2) production is the direct inhibition of COX enzymatic activity.


