StepharineCAS# 2810-21-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2810-21-1 | SDF | Download SDF |
| PubChem ID | 98455 | Appearance | Powder |
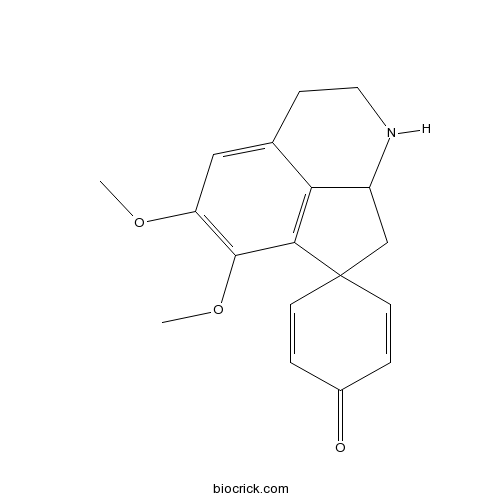
| Formula | C18H19NO3 | M.Wt | 297.3 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 10,11-dimethoxyspiro[5-azatricyclo[6.3.1.04,12]dodeca-1(12),8,10-triene-2,4'-cyclohexa-2,5-diene]-1'-one | ||
| SMILES | COC1=C(C2=C3C(CC24C=CC(=O)C=C4)NCCC3=C1)OC | ||
| Standard InChIKey | OGJKMZVUJJYWKO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H19NO3/c1-21-14-9-11-5-8-19-13-10-18(6-3-12(20)4-7-18)16(15(11)13)17(14)22-2/h3-4,6-7,9,13,19H,5,8,10H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Stepharine Dilution Calculator

Stepharine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.3636 mL | 16.818 mL | 33.6361 mL | 67.2721 mL | 84.0901 mL |
| 5 mM | 0.6727 mL | 3.3636 mL | 6.7272 mL | 13.4544 mL | 16.818 mL |
| 10 mM | 0.3364 mL | 1.6818 mL | 3.3636 mL | 6.7272 mL | 8.409 mL |
| 50 mM | 0.0673 mL | 0.3364 mL | 0.6727 mL | 1.3454 mL | 1.6818 mL |
| 100 mM | 0.0336 mL | 0.1682 mL | 0.3364 mL | 0.6727 mL | 0.8409 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 7,7'-Dihydrotaiwanin C
Catalog No.:BCN9604
CAS No.:216955-79-2
- Menisdaurilide
Catalog No.:BCN9603
CAS No.:67765-59-7
- Aquilegiolide
Catalog No.:BCN9602
CAS No.:94481-79-5
- 16-Oxoserratenediol
Catalog No.:BCN9601
CAS No.:24513-52-8
- Norushinsunine
Catalog No.:BCN9600
CAS No.:3175-84-6
- 3β-Hydroxy-7β,25-dimethoxycucurbita-5,23-dien-19-al
Catalog No.:BCN9599
CAS No.:85372-69-6
- (2R,3R)-Glucodistylin
Catalog No.:BCN9598
CAS No.:27297-45-6
- Sinococuline
Catalog No.:BCN9597
CAS No.:109351-36-2
- Tannagine
Catalog No.:BCN9596
CAS No.:123750-34-5
- Junosine
Catalog No.:BCN9595
CAS No.:103956-34-9
- Citrusinine I
Catalog No.:BCN9594
CAS No.:86680-32-2
- 6a,7-Dehydroboldine
Catalog No.:BCN9593
CAS No.:91599-23-4
- Taiwanin C
Catalog No.:BCN9606
CAS No.:14944-34-4
- Beiwutine
Catalog No.:BCN9607
CAS No.:76918-93-9
- Apigenin 7-O-(2'',6''-di-O-E-p-coumaroyl)glucoside
Catalog No.:BCN9608
CAS No.:1448779-19-8
- Karavilagenin F
Catalog No.:BCN9609
CAS No.:1639024-15-9
- 3-O-(p-Hydroxybenzoyl)serratriol
Catalog No.:BCN9610
CAS No.:1448534-93-7
- 5-Methoxyjusticidin A
Catalog No.:BCN9611
CAS No.:205505-62-0
- Taiwanin E
Catalog No.:BCN9612
CAS No.:22743-05-1
- 6,7-Di-O-acetylsinococuline
Catalog No.:BCN9613
CAS No.:1054312-81-0
- Illiciumlignan D
Catalog No.:BCN9614
CAS No.:2237239-36-8
- Tuberculatin
Catalog No.:BCN9615
CAS No.:90706-10-8
- Greveichromenol
Catalog No.:BCN9616
CAS No.:35930-29-1
- Cernuine
Catalog No.:BCN9617
CAS No.:6880-84-8
Tempo-Spatial Pattern of Stepharine Accumulation in Stephania Glabra Morphogenic Tissues.[Pubmed:30781887]
Int J Mol Sci. 2019 Feb 13;20(4). pii: ijms20040808.
Alkaloids attract great attention due to their valuable therapeutic properties. Stepharine, an aporphine alkaloid of Stephania glabra plants, exhibits anti-aging, anti-hypertensive, and anti-viral effects. The distribution of aporphine alkaloids in cell cultures, as well as whole plants is unknown, which hampers the development of bioengineering strategies toward enhancing their production. The spatial distribution of Stepharine in cell culture models, plantlets, and mature micropropagated plants was investigated at the cellular and organ levels. Stepharine biosynthesis was found to be highly spatially and temporally regulated during plant development. We proposed that self-intoxication is the most likely reason for the failure of the induction of alkaloid biosynthesis in cell cultures. During somatic embryo development, the toxic load of alkaloids inside the cells increased. Only specialized cell sites such as vascular tissues with companion cells (VT cells), laticifers, and parenchymal cells with inclusions (PI cells) can tolerate the accumulation of alkaloids, and thus circumvent this restriction. S. glabra plants have adapted to toxic pressure by forming an additional transport secretory (laticifer) system and depository PI cells. Postembryonic growth restricts specialized cell site formation during organ development. Future bioengineering strategies should include cultures enriched in the specific cells identified in this study.
Isoquinoline alkaloids from Asimina triloba.[Pubmed:30453785]
Nat Prod Res. 2019 Oct;33(19):2823-2829.
A new aporphine glycoside, (-)-anolobine-9-O-beta-D-glucopyranoside was isolated from the twigs of pawpaw (Asimina triloba) along with 7 known alkaloids including five aporphine alkaloids (anolobine, nornuciferine, norushinsunine, liriodenine, and lysicamine), a proaporhine alkaloid (Stepharine), and a tetrahydrobenzylisoquinoline alkaloid (coclaurine). Among these compounds, nornuciferine, lysicamine, Stepharine, and coclaurine are reported for the first time from this plant. The structure of the new compound was elucidated by spectroscopic methods, including 1 D, 2 D NMR, and HRESI-MS. The absolute configuration of compounds 1, 2, 7 and 8 was determined by CD experiment.
Development of an HPLC-DAD method for the determination of five alkaloids in Stephania yunnanensis Lo and in rat plasma after oral dose of Stephania yunnanensis Lo extracts.[Pubmed:29782649]
Biomed Chromatogr. 2018 Oct;32(10):e4292.
For the rational utilization and the quantitative quality control of the Stephania yunnanensis Lo, an HPLC-DAD method was developed for the quantitative and simultaneous determination of five alkaloids in rat plasma (Stepharine, sinomenine, palmatine, isocorydine and tetrahydropalmatine), which were the main active chemical constituents of this plant and belong to four kinds of isoquinoline-type alkaloids (protoberberine, morphine, aporphine and protaporphine alkaloids). The contents of five alkaloids ranged from 0.09 to 2.32% (w/w). The method validation was tested for the linearity (r(2) > 0.9975), precision (intra-day RSD < 4.8% and inter-day RSD < 4.9%), extraction recovery (85.49 +/- 2.29% to 99.21 +/- 1.48%) and stability (98.5 +/- 5.3% to 101.2 +/- 3.4%). We developed an HPLC-DAD method to simultaneously measure these alkaloids in rat plasma after oral administration of the extract of this plant to rats. The results supported the hypothesis that isoquinoline alkaloids were the compounds responsible for the main pharmacological activities for anti-inflammatory and analgesic.
Chemical profiling of the tuber of Stephania cambodica Gagnep. (Menispermaceae) and analytical control by UHPLC-DAD.[Pubmed:27976592]
Nat Prod Res. 2017 Apr;31(7):802-809.
A new aporphine glycoside (1), named 'angkorwatine', and eight known alkaloids: oblongine (2), Stepharine (3), asimilobine-beta-d-glucopyranoside (4), isocorydine (5), tetrahydropalmatine (THP) (6), jatrorrhizine (7), palmatine (PAL) (8), and roemerine (ROE) (9) were simultaneously isolated from the tuber of Stephania cambodica. The development and validation of UHPLC-DAD method was carried out for the quantification of marker compounds (PAL, ROE, THP) of S. cambodica. In addition to good selectivity and linearity (r(2) > 0.997), trueness, precision, and accuracy of the method did not exceed the acceptance limit of +/-10% for ROE, THP and +/-20% for PAL. Consequently, this method is able to provide accurate results between 1.39-4.18 mug/mL, 2.01-30.72 mug/mL, and 4.29-64.42 mug/mL for PAL, ROE, and THP, respectively. This study shows that the validated UHPLC method is a rapid, innovative and effective analytical approach to control quality of tubers of S. cambodica and to regulate the usage of this plant in traditional medicine.
Development of mass spectrometry selected reaction monitoring method for quantitation and pharmacokinetic study of stepharine in rabbit plasma.[Pubmed:24696679]
Adv Pharmacol Sci. 2014;2014:961850.
Highly sensitive liquid chromatography mass spectrometry method on triple quadrupole (QQQ) mass spectrometer was successfully applied for pharmacokinetic study of Stepharine in rabbit plasma. Specific ion transitions of Stepharine protonated precursor ion were selected and recorded in the certain retention time employing dynamic selected reaction monitoring mode. The developed method facilitated quantitative measurements of Stepharine in plasma samples in linear range of five orders of magnitude with high accuracy and low standard deviation coefficient and pharmacokinetics parameters were calculated. The apparent volume of Stepharine distribution (estimated as ratio of clearance to elimination rate constant, data not shown) allows us to assume that Stepharine was extensively distributed throughout the body.
[Akaloids from roots of Stephania dentifolia].[Pubmed:23713286]
Zhongguo Zhong Yao Za Zhi. 2013 Feb;38(4):574-7.
Eight alkaloids were isolated from the thin sulfuric acid extracts of the fresh roots of Stephania dentifolia by aluminum oxide, silica and Sephadex LH-20 column chromatography methods. Based on the spectroscopic analysis and chemical evidence, the structures of these alkaloids were identified as sinoacutine (1), sinomenine (2), cephamonine (3), tetrahydropalmatine (4), capaurine (5), stepharanine (6), (+)-Stepharine (7) and palmatine (8). All compounds were obtained from this plant for the first time.
Comprehensive separation and analysis of alkaloids from Stephania yunnanensis by counter-current chromatography coupled with liquid chromatography tandem mass spectrometry analysis.[Pubmed:22041142]
J Chromatogr A. 2012 Feb 24;1226:18-23.
The polar compounds such as alkaloid compounds are important bioactive components in traditional Chinese medicines. In present study, a comprehensive method for separation and analysis of polar compounds from the polar fraction of traditional Chinese medicine Stephania yunnanensis was established. Both the major components and minor components were analyzed by counter-current chromatography combined with liquid chromatography tandem mass spectrometry (LC-MS(n)). From 50 mg polar fraction of crude extract, 15.2mg corydine and 4.8 mg Stepharine with purities over 90% were successfully separated via a polar solvent system n-butanol: methanol: water (4:1:5, v/v) with 10 mM NaOH as an additive in the lower phase, in one step operation. Their structures were further identified by 1H NMR and FTICR-MS. Besides, three minor components were identified by HPLC-MS(n) based on the fragmentation behavior of the purified compounds.
Aporphine alkaloids from the roots of Stephania viridiflavens.[Pubmed:20645211]
Nat Prod Res. 2010 Aug;24(13):1243-7.
Further investigation on the phytochemistry of the medicinal plant Stephania viridiflavens led to the isolation of a new naturally occurring aporphine alkaloid, (+)-6R, 6aS-isocorydine N(beta)-oxide (1), together with three known aporphine alkaloids: (+)-6R, 6aS-corydine N(beta)-oxide (2), (+)-N-methyl-laurotetanine (3) and (+)-Stepharine (4). The structure and stereochemistry of 1 were determined on the basis of spectroscopic methods and confirmed by synthesis. Alkaloids 2-4 were isolated for the first time from this species.
Novel and efficient synthetic path to proaporphine alkaloids: total synthesis of (+/-)-stepharine and (+/-)-pronuciferine.[Pubmed:16468735]
Org Lett. 2006 Feb 16;8(4):657-9.
[reaction: see text] A novel synthetic path to proaporphine alkaloids was established by employing aromatic oxidation with a hypervalent iodine reagent, where an unprecedented carbon-carbon bond forming reaction between the para-position of a phenol group and an enamide-carbon took place smoothly to give the desired spiro-cyclohexadienone.
[Alkaloids of Annonaceae. XXIX. Alkaloids of Annona muricata].[Pubmed:17401878]
Planta Med. 1981 May;42(1):37-44.
From leaves, root - and stem - barks of Annona muricata L., seven isoquinoline alkaloids have been isolated: reticuline (main alkaloid), coclaurine, coreximine, atherosperminine, Stepharine. Anomurine and anomuricine, two minor alkaloids, are new tetrahydrobenzylisoquinolines, with 5, 6, 7 substituted ring A. The phytochemical significance of these alkaloids is discussed.


