SalvadorasideCAS# 143522-30-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 143522-30-9 | SDF | Download SDF |
| PubChem ID | 162343330.0 | Appearance | Powder |
| Formula | C34H48O18 | M.Wt | 744.74 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
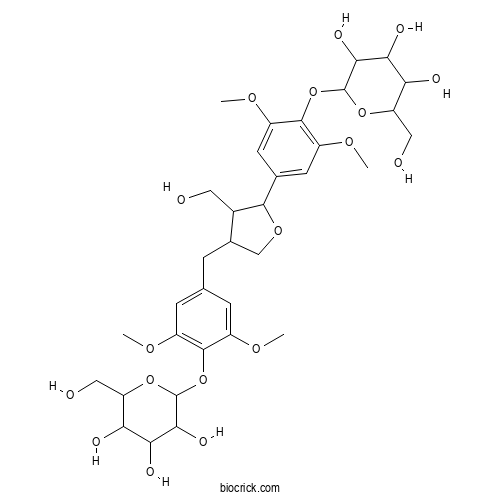
| Chemical Name | 2-[4-[[5-[3,5-dimethoxy-4-[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]-4-(hydroxymethyl)oxolan-3-yl]methyl]-2,6-dimethoxyphenoxy]-6-(hydroxymethyl)oxane-3,4,5-triol | ||
| SMILES | COC1=CC(=CC(=C1OC2C(C(C(C(O2)CO)O)O)O)OC)CC3COC(C3CO)C4=CC(=C(C(=C4)OC)OC5C(C(C(C(O5)CO)O)O)O)OC | ||
| Standard InChIKey | JJHDIHQKWJEDDR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C34H48O18/c1-44-18-6-14(7-19(45-2)31(18)51-33-28(42)26(40)24(38)22(11-36)49-33)5-16-13-48-30(17(16)10-35)15-8-20(46-3)32(21(9-15)47-4)52-34-29(43)27(41)25(39)23(12-37)50-34/h6-9,16-17,22-30,33-43H,5,10-13H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Salvadoraside Dilution Calculator

Salvadoraside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.3428 mL | 6.7138 mL | 13.4275 mL | 26.855 mL | 33.5688 mL |
| 5 mM | 0.2686 mL | 1.3428 mL | 2.6855 mL | 5.371 mL | 6.7138 mL |
| 10 mM | 0.1343 mL | 0.6714 mL | 1.3428 mL | 2.6855 mL | 3.3569 mL |
| 50 mM | 0.0269 mL | 0.1343 mL | 0.2686 mL | 0.5371 mL | 0.6714 mL |
| 100 mM | 0.0134 mL | 0.0671 mL | 0.1343 mL | 0.2686 mL | 0.3357 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Betulone
Catalog No.:BCX1251
CAS No.:7020-34-0
- D-Gluconic acid, 6-[(2E)-3-(3,4-dihydroxyphenyl)-2-propenoate]
Catalog No.:BCX1250
CAS No.:1147861-80-0
- 11(α)-Hydroxynepasaikosaponin k
Catalog No.:BCX1249
CAS No.:1152168-63-2
- 16β-Hydroperoxyalisol B 23-acetate
Catalog No.:BCX1248
CAS No.:2221029-54-3
- trans-Ferulic acid-4-β-glucoside
Catalog No.:BCX1247
CAS No.:117405-51-3
- Carmichasine B
Catalog No.:BCX1246
CAS No.:2245700-60-9
- Hypoletin-7-O-β-D-xylopyranoside
Catalog No.:BCX1245
CAS No.:126771-28-6
- Hypolaetin 7-O-glucoside
Catalog No.:BCX1244
CAS No.:32455-43-9
- Aconicarchamine B
Catalog No.:BCX1243
CAS No.:1275535-67-5
- Damnacanthol
Catalog No.:BCX1242
CAS No.:477-83-8
- Chikusaikoside II
Catalog No.:BCX1241
CAS No.:166338-14-3
- 1-O-gentiobiosyl-3,7-dimethoxy-8-hydroxyxanthone
Catalog No.:BCX1240
CAS No.:487040-33-5
- Ophiopogonone C
Catalog No.:BCX1253
CAS No.:477336-77-9
- Casuarictin
Catalog No.:BCX1254
CAS No.:79786-00-8
- Ophiopogonanone D
Catalog No.:BCX1255
CAS No.:1027912-99-7
- Tenaxin I
Catalog No.:BCX1256
CAS No.:86926-52-5
- Tibesaikosaponin V
Catalog No.:BCX1257
CAS No.:2319668-87-4
- Stipuleanoside R2
Catalog No.:BCX1258
CAS No.:96627-72-4
- Brevicornin
Catalog No.:BCX1259
CAS No.:173792-49-9
- cis-Pellitorine
Catalog No.:BCX1260
CAS No.:639086-18-3
- Tenacissoside A
Catalog No.:BCX1261
CAS No.:107352-30-7
- Isoasiaticoside
Catalog No.:BCX1262
CAS No.:948827-09-6
- Fallacinol
Catalog No.:BCX1263
CAS No.:569-05-1
- Tarasaponin IV
Catalog No.:BCX1264
CAS No.:156980-31-3
Oenothera biennis cell culture produce lignans activating Piezo1 triggering the Myosin Light Chain Kinase depending pathways.[Pubmed:37748257]
Biochem Biophys Res Commun. 2023 Nov 12;681:36-40.
Piezo1 and Piezo2 are mechanoreceptors involved in sensing both internal and external mechanical forces converting them in electrical signals to the brain. Piezo1 is mainly expressed in the endothelial system and in epidermis sensing shear stress and light touch. The internal traction forces generated by Myosin Light Chain Kinase (MYLK) activate Piezo1, regulating cell contraction. We observed Oenothera biennis cell culture hydro-soluble extract (ObHEx) activated MYLK regulating cell contraction ability. The aim of this work was to test the hypothesis that ObHEx activates Piezo1 through MYLK pathway using CHO cell overexpressing Piezo1, HUVEC and SHSY5Y cells endogenously expressing high levels of Piezo1. Results showed that ObHEx extracts were able to activate Piezo1 and the effect is due to Liriodendrin and Salvadoraside, the two most abundant lignans produced by the cell culture. The effect is lost in presence of MYLK specific inhibitors confirming the key role of this pathway and providing indication about the mechanism of action in Piezo1 activation by lignans. In summary, these results confirmed the connection between Piezo1 and MYLK, opening the possibility of using lignans-containing natural extracts to activate Piezo1.
[Investigation of the chemical components of Ciwujia injection using ultra-high performance liquid chromatography-quadrupole-electrostatic field orbitrap high-resolution mass spectrometry].[Pubmed:36861204]
Se Pu. 2023 Mar;41(3):207-223.
Ciwujia injection is commonly used to treat cerebrovascular and central nervous system diseases in clinical practice. It can significantly improve blood lipid levels and endothelial cell function in patients with acute cerebral infarction and promote the proliferation of neural stem cells in cerebral ischemic brain tissues. The injection has also been reported to have good curative effects on cerebrovascular diseases, such as hypertension and cerebral infarction. At present, the material basis of Ciwujia injection remains incompletely understood, and only two studies have reported dozens of components, which were determined using high performance liquid chromatography-quadrupole time-of-flight mass spectrometry (HPLC-Q-TOF MS). Unfortunately, the lack of research on this injection restricts the in-depth study of its therapeutic mechanism.In the present study, a qualitative method based on ultra-high performance liquid chromatography-quadrupole-electrostatic field orbitrap high-resolution mass spectrometry (UHPLC-Q/Orbitrap HRMS) was developed to analyze the chemical components of Ciwujia injection. Separation was performed on a BEH Shield RP18 column (100 mmx2.1 mm, 1.7 mum) using 0.1% formic acid aqueous solution (A) and acetonitrile (B) as the mobile phases, and gradient elution was performed as follows: 0-2 min, 0%B; 2-4 min, 0%B-5%B; 4-15 min, 5%B-20%B; 15-15.1 min, 20%B-90%B; 15.1-17 min, 90%B. The flow rate and column temperature were set to 0.4 mL/min and 30 ℃ respectively. MS(1) and MS(2) data were acquired in both positive- and negative-ion modes using a mass spectrometer equipped with an HESI source. For data post-processing, a self-built library including component names, molecular formulas, and chemical structures was established by collecting information on the isolated chemical compounds of Acanthopanax senticosus. The chemical components of the injection were identified by comparison with standard compounds or MS(2) data in commercial databases or literature based on precise relative molecular mass and fragment ion information. The fragmentation patterns were also considered. For example, the MS(2) data of 3-caffeoylquinic acid (chlorogenic acid), 4-caffeoylquinic acid (cryptochlorogenic acid), and 5-caffeoylquinic acid (neochlorogenic acid) were first analyzed. The results indicated that these compounds possessed similar fragmentation behaviors, yielding product ions at m/z 173 and m/z 179 simultaneously. However, the abundance of the product ion at m/z 173 was much higher in 4-caffeoylquinic acid than in 5-caffeoylquinic acid or 3-caffeoylquinic acid, and the fragment signal at m/z 179 was much stronger for 5-caffeoylquinic acid than for 3-caffeoylquinic acid. Four caffeoylquinic acids were identified using a combination of abundance information and retention times. MS(2) data in commercial database and literature were also used to identify unknown constituents. For example, compound 88 was successfully identified as possessing a relative molecular mass and neutral losses similar to those of sinapaldehyde using the database, and compound 80 was identified as Salvadoraside because its molecular and fragmentation behaviors were consistent with those reported in the literature. A total of 102 constituents, including 62 phenylpropanoids, 23 organic acids, 7 nucleosides, 1 iridoid, and 9 other compounds, were identified. The phenylpropanoids can be further classified as phenylpropionic acids, phenylpropanols, benzenepropanals, coumarins, and lignans. Among the detected compounds, 16 compounds were confirmed using reference compounds and 65 compounds were identified in Ciwujia injection for the first time. This study is the first to report the feasibility of using the UHPLC-Q/Orbitrap HRMS method to quickly and comprehensively analyze the chemical components of Ciwujia injection. The 27 newly discovered phenylpropanoids provide further material basis for the clinical treatment of neurological diseases and new research targets for the in-depth elucidation of the pharmacodynamic mechanism of Ciwujia injection and its related preparations.
Inhibition of Lung Inflammation by Acanthopanax divaricatus var. Albeofructus and Its Constituents.[Pubmed:26759704]
Biomol Ther (Seoul). 2016 Jan;24(1):67-74.
In order to find potential therapeutic agents on lung inflammatory conditions, the extracts of Acanthopanax divaricatus var. albeofructus were prepared and its constituents were isolated. They include lignans such as (+)-syringaresinol (1), acanthoside B (2), Salvadoraside (3) and acanthoside D (4), lariciresinol-9-O-beta-D-glucopyranoside (5) and phenylpropanoids such as 4-[(1E)-3-methoxy-1-propenyl]phenol (6), coniferin (7), and methyl caffeate (8). The extracts and several constituents such as compound 1, 6 and 8 inhibited the production of inflammatory markers, IL-6 and nitric oxide, from IL-1beta-treated lung epithelial cells and lipopolysaccharide (LPS)-treated alveolar macrophages. Furthermore, the extracts and compound 4 significantly inhibited lung inflammation in lipolysaccharide-treated acute lung injury in mice by oral administration. Thus it is suggested that A. divaricatus var. albeofructus and its several constituents may be effective against lung inflammation.


