CasuarictinCAS# 79786-00-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 79786-00-8 | SDF | Download SDF |
| PubChem ID | 73644.0 | Appearance | Powder |
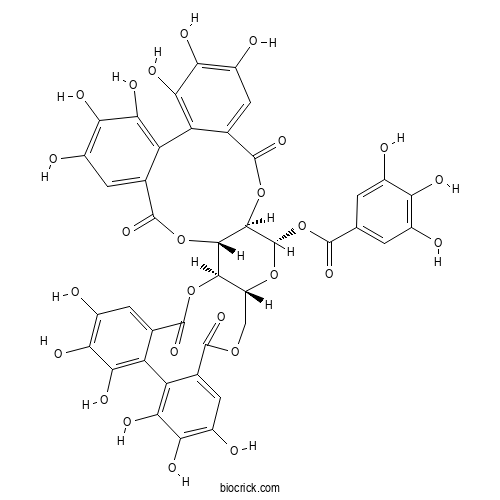
| Formula | C41H28O26 | M.Wt | 936.65 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(1R,2S,19R,20S,22R)-7,8,9,12,13,14,28,29,30,33,34,35-dodecahydroxy-4,17,25,38-tetraoxo-3,18,21,24,39-pentaoxaheptacyclo[20.17.0.02,19.05,10.011,16.026,31.032,37]nonatriaconta-5,7,9,11,13,15,26,28,30,32,34,36-dodecaen-20-yl] 3,4,5-trihydroxybenzoate | ||
| SMILES | C1C2C(C3C(C(O2)OC(=O)C4=CC(=C(C(=C4)O)O)O)OC(=O)C5=CC(=C(C(=C5C6=C(C(=C(C=C6C(=O)O3)O)O)O)O)O)O)OC(=O)C7=CC(=C(C(=C7C8=C(C(=C(C=C8C(=O)O1)O)O)O)O)O)O | ||
| Standard InChIKey | SWRFKGRMQVLMKA-JIZJWZDPSA-N | ||
| Standard InChI | InChI=1S/C41H28O26/c42-13-1-8(2-14(43)24(13)48)36(57)67-41-35-34(65-39(60)11-5-17(46)27(51)31(55)22(11)23-12(40(61)66-35)6-18(47)28(52)32(23)56)33-19(63-41)7-62-37(58)9-3-15(44)25(49)29(53)20(9)21-10(38(59)64-33)4-16(45)26(50)30(21)54/h1-6,19,33-35,41-56H,7H2/t19-,33-,34+,35-,41+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Casuarictin Dilution Calculator

Casuarictin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.0676 mL | 5.3382 mL | 10.6763 mL | 21.3527 mL | 26.6909 mL |
| 5 mM | 0.2135 mL | 1.0676 mL | 2.1353 mL | 4.2705 mL | 5.3382 mL |
| 10 mM | 0.1068 mL | 0.5338 mL | 1.0676 mL | 2.1353 mL | 2.6691 mL |
| 50 mM | 0.0214 mL | 0.1068 mL | 0.2135 mL | 0.4271 mL | 0.5338 mL |
| 100 mM | 0.0107 mL | 0.0534 mL | 0.1068 mL | 0.2135 mL | 0.2669 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ophiopogonone C
Catalog No.:BCX1253
CAS No.:477336-77-9
- Salvadoraside
Catalog No.:BCX1252
CAS No.:143522-30-9
- Betulone
Catalog No.:BCX1251
CAS No.:7020-34-0
- D-Gluconic acid, 6-[(2E)-3-(3,4-dihydroxyphenyl)-2-propenoate]
Catalog No.:BCX1250
CAS No.:1147861-80-0
- 11(α)-Hydroxynepasaikosaponin k
Catalog No.:BCX1249
CAS No.:1152168-63-2
- 16β-Hydroperoxyalisol B 23-acetate
Catalog No.:BCX1248
CAS No.:2221029-54-3
- trans-Ferulic acid-4-β-glucoside
Catalog No.:BCX1247
CAS No.:117405-51-3
- Carmichasine B
Catalog No.:BCX1246
CAS No.:2245700-60-9
- Hypoletin-7-O-β-D-xylopyranoside
Catalog No.:BCX1245
CAS No.:126771-28-6
- Hypolaetin 7-O-glucoside
Catalog No.:BCX1244
CAS No.:32455-43-9
- Aconicarchamine B
Catalog No.:BCX1243
CAS No.:1275535-67-5
- Damnacanthol
Catalog No.:BCX1242
CAS No.:477-83-8
- Ophiopogonanone D
Catalog No.:BCX1255
CAS No.:1027912-99-7
- Tenaxin I
Catalog No.:BCX1256
CAS No.:86926-52-5
- Tibesaikosaponin V
Catalog No.:BCX1257
CAS No.:2319668-87-4
- Stipuleanoside R2
Catalog No.:BCX1258
CAS No.:96627-72-4
- Brevicornin
Catalog No.:BCX1259
CAS No.:173792-49-9
- cis-Pellitorine
Catalog No.:BCX1260
CAS No.:639086-18-3
- Tenacissoside A
Catalog No.:BCX1261
CAS No.:107352-30-7
- Isoasiaticoside
Catalog No.:BCX1262
CAS No.:948827-09-6
- Fallacinol
Catalog No.:BCX1263
CAS No.:569-05-1
- Tarasaponin IV
Catalog No.:BCX1264
CAS No.:156980-31-3
- Dihydrolanosterol
Catalog No.:BCX1265
CAS No.:911660-54-3
- Araloside C
Catalog No.:BCX1266
CAS No.:55446-15-6
Pharmacological Property and Cytotoxic Effect Showing Antiproliferative Potency in Human Melanoma Cell Lines (A375) of Combretum racemosum P. Beauv. Leaf and Root Extracts Used in Benin.[Pubmed:38247456]
Antioxidants (Basel). 2023 Dec 22;13(1):31.
Combretum racemosum, a plant from the Combretaceae family, is traditionally used in Benin for various health problems. However, scientific research on Beninese samples of this plant is limited. The aim of this study was to identify and assess the bioactive compounds in the plant's leaves and roots. Initial screening involved analyzing powders derived from these parts for total polyphenols, flavonoids, and both condensed and hydrolyzable tannins. The polyphenolic compounds were analyzed using HPLC-DAD-ESI-MS. To evaluate the plant's antimicrobial properties, the agar diffusion method was employed, while FRAP and DPPH assays were used to determine its antioxidant capacity. For anti-inflammatory activity, the study utilized tests for in vitro protein denaturation inhibition and in vivo acute edema induced by carrageenan. Additionally, an antiproliferative assay was conducted using the human melanoma cell line A375. The analysis revealed the presence of significant polyphenolic compounds in both the leaf and root extracts of C. racemosum. Notably, compounds like Pedunculagin, Vescalagin, Casuarictin, and Digalloyl-glucoside were abundant in the leaves, with Vescalagin being especially predominant in the roots. The study also found that the dichloromethane extracts from the leaves and roots exhibited bactericidal effects on a substantial percentage of meat-isolated strains. Moreover, the antioxidant activities of these extracts were confirmed through FRAP and DPPH methods. Interestingly, the dichloromethane root extract showed strong activity in inhibiting thermal albumin denaturation, while the water-ethanol leaf extract demonstrated significant edema inhibition. Finally, the study observed that C. racemosum extracts reduced cell viability in a dose-dependent manner, with leaf extracts showing more pronounced antiproliferative effects than root extracts. These findings highlight the potential of C. racemosum leaves and roots as sources of compounds with diverse and significant biological activities. In conclusion, C. racemosum's leaves and roots exhibit promising biological activities, highlighting their potential medicinal value.
Metabolites of Geum aleppicum and Sibbaldianthe bifurca: Diversity and alpha-Glucosidase Inhibitory Potential.[Pubmed:37367847]
Metabolites. 2023 May 25;13(6):689.
alpha-Glucosidase inhibitors are essential in the treatment of diabetes mellitus. Plant-derived drugs are promising sources of new compounds with glucosidase-inhibiting ability. The Geum aleppicum Jacq. and Sibbaldianthe bifurca (L.) Kurtto & T.Erikss. herbs are used in many traditional medical systems to treat diabetes. In this study, metabolites of the G. aleppicum and S. bifurca herbs in active growth, flowering, and fruiting stages were investigated using high-performance liquid chromatography with photodiode array and electrospray ionization triple quadrupole mass spectrometric detection (HPLC-PDA-ESI-tQ-MS/MS). In total, 29 compounds in G. aleppicum and 41 components in S. bifurca were identified including carbohydrates, organic acids, benzoic and ellagic acid derivatives, ellagitannins, flavonoids, and triterpenoids. Gemin A, miquelianin, niga-ichigoside F1, and 3,4-dihydroxybenzoic acid 4-O-glucoside were the dominant compounds in the G. aleppicum herb, while guaiaverin, miquelianin, tellimagrandin II(2), Casuarictin, and glucose were prevailing compounds in the S. bifurca herb. On the basis of HPLC activity-based profiling of the G. aleppicum herb extract, the most pronounced inhibition of alpha-glucosidase was observed for gemin A and quercetin-3-O-glucuronide. The latter compound and quercetin-3-O-arabinoside demonstrated maximal inhibition of alpha-glucosidase in the S. bifurca herb extract. The obtained results confirm the prospects of using these plant compounds as possible sources of hypoglycemic nutraceuticals.
Anti-inflammatory activities of black raspberry seed ellagitannins and their structural effects on the stimulation of glucagon-like peptide-1 secretion and intestinal bitter taste receptors.[Pubmed:37073737]
Food Funct. 2023 May 11;14(9):4049-4064.
This study aimed to investigate the anti-inflammatory effects of ellagitannins from black raspberry seeds (BS) in vivo and the structural effects of ellagitannins on glucagon-like peptide-1 (GLP-1) secretion and intestinal bitter taste receptor (TAS2R) stimulation. For animal study, BS ellagitannin fraction (BSEF) was orally administered to mice with colitis induced by dextran sulfate sodium (DSS). The BSEF supplementation alleviated colonic inflammation, regulated inflammation-related cytokine levels in the mice with colitis, and increased the total GLP-1 secretion and GLP-1 receptor mRNA level in the inflamed gut. It also augmented the colonic gene expressions of mouse TAS2R (mTAS2R) 108, 119, 126, 131, 138, and 140; meanwhile, only mTAS2R108 expression was downregulated by DSS treatment. Six BS ellagitannins (sanguiin H-6, Casuarictin, pedunculagin, acutissimin A, castalagin, and vescalagin) induced GLP-1 secretion in STC-1 cells and upregulated mTAS2R108, 119, 126, and 138 gene expressions. The major ellagitannins in BS (sanguiin H-6, Casuarictin, pedunculagin, and acutissimin A) upregulated the gene expressions of mTAS2R131 and/or 140 known to be specifically distributed in mouse colon. Through molecular docking with mTAS2R108, the hexahydroxydiphenoyl, flavan-3-ol, glucose, and nonahydroxytriphenoyl moieties of the six BS ellagitannins were predicted to be involved in interacting with the receptor. BS ellagitannins could be promising candidates for preventing colon inflammation, likely via GLP-1 secretion induced by intestine-specific TAS2Rs.
Identification of natural compounds as SARS-CoV-2 inhibitors via molecular docking and molecular dynamic simulation.[Pubmed:36817101]
Front Microbiol. 2023 Feb 1;13:1095068.
BACKGROUND: Base mutations increase the contagiousness and transmissibility of the Delta and Lambda strains and lead to the severity of the COVID-19 pandemic. Molecular docking and molecular dynamics (MD) simulations are frequently used for drug discovery and relocation. Small molecular compounds from Chinese herbs have an inhibitory effect on the virus. Therefore, this study used computational simulations to investigate the effects of small molecular compounds on the spike (S) protein and the binding between them and angiotensin-converting enzyme 2 (ACE2) receptors. METHODS: In this study, molecular docking, MD simulation, and protein-protein analysis were used to explore the medicinal target inhibition of Chinese herbal medicinal plant chemicals on SARS-CoV-2. 12,978 phytochemicals were screened against S proteins of SARS-CoV-2 Lambda and Delta mutants. RESULTS: Molecular docking showed that 65.61% and 65.28% of the compounds had the relatively stable binding ability to the S protein of Lambda and Delta mutants (docking score Casuarictin, Heterophylliin D, Protohypericin, and Glansrin B) could interact with S protein mutation sites of Lambda and Delta mutants, respectively, and MD simulation results showed that four plant chemicals and spike protein have good energy stable complex formation ability. In addition, protein-protein docking was carried out to evaluate the changes in ACE2 binding ability caused by the formation of four plant chemicals and S protein complexes. The analysis showed that the binding of four plant chemicals to the S protein could reduce the stability of the binding to ACE2, thereby reducing the replication ability of the virus. CONCLUSION: To sum up, the study concluded that four phytochemicals (Casuarictin, Heterophylliin D, Protohypericin, and Glansrin B) had significant effects on the binding sites of the SARS-CoV-2 S protein. This study needs further in vitro and in vivo experimental validation of these major phytochemicals to assess their potential anti-SARS-CoV-2. Graphical abstract.
Inhibitory Effects of Hydrolysable Tannins on Lipid Accumulation in 3T3-L1 Cells.[Pubmed:36184503]
Biol Pharm Bull. 2022;45(10):1458-1465.
Obesity is currently the most common cause of metabolic diseases including type 2 diabetes and hyperlipidemia. Obesity results from excess lipid accumulation in adipose tissue. Several studies have investigated the inhibitory effects of natural plant-derived products on adipocyte differentiation and lipid accumulation. In this study, we examined the effect of hydrolysable tannins composed of gallic acid and glucose on adipocyte differentiation in 3T3-L1 cells. 1,2,3,4,6-Penta-O-galloyl-beta-D-glucose (PGG) (1), a representative gallotannin, inhibited lipid accumulation in 3T3-L1 cells, whereas ellagitannins (tellimagrandin I, eugeniin and Casuarictin) did not. The expression of adipocyte differentiation-related genes, including peroxisome proliferator activator gamma2 (Ppargamma2), CCAAT/enhancer binding protein alpha (C/EBPalpha) and adipocyte fatty acid binding protein (aP2), was significantly suppressed in PGG (1)-treated 3T3-L1 cells beginning at day 2 after induction of differentiation. While PGG (1) did not directly reduce Ppargamma2 expression, it reduced the expression of its target genes in mature adipocytes. In addition, PGG (1) treatment inhibited mitotic clonal expansion, one of earliest events of adipocyte differentiation. These findings indicate that PGG (1) has an inhibitory effect on adipocyte differentiation through the suppression of mitotic clonal expansion.
Identification of a novel alpha-glucosidase inhibitor from Melastoma dodecandrum Lour. fruits and its effect on regulating postprandial blood glucose.[Pubmed:36037688]
Food Chem. 2023 Jan 15;399:133999.
Melastoma dodecandrum Lour. (MDL) extracts have shown potent alpha-glucosidase inhibitory activity, suggesting MDL might be a good source of alpha-glucosidase inhibitors. The aim of the study was to identify compounds in MDL extracts with alpha-glucosidase inhibitory activities and evaluate their effect on postprandial blood glucose as well as elucidating the underlying mechanisms of inhibition. A total of 34 polyphenols were identified in MDL fruits, among which 10 anthocyanins and three proanthocyanidin derivatives were discovered for the first time. Dosing mice with MDL extracts (100 mg/kg body weight, by gavage) was associated with a significantly decrease in postprandial blood glucose concentrations after oral administration of maltose. The most potent alpha-glucosidase inhibitor was identified as Casuarictin (IC(50) of 0.21 mug/mL). Casuarictin bound competitively to alpha-glucosidase, occupying not only the catalytic site but also forming strong hydrogen bonds with alpha-glucosidase residues. Therefore, Casuarictin derived from MDL fruits might be used as novel alpha-glucosidase inhibitor in functional foods or other dietary products.
Evaluation of Clove Phytochemicals as Potential Antiviral Drug Candidates Targeting SARS-CoV-2 Main Protease: Computational Docking, Molecular Dynamics Simulation, and Pharmacokinetic Profiling.[Pubmed:35836934]
Front Mol Biosci. 2022 Jun 28;9:918101.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus can cause a sudden respiratory disease spreading with a high mortality rate arising with unknown mechanisms. Still, there is no proper treatment available to overcome the disease, which urges the research community and pharmaceutical industries to screen a novel therapeutic intervention to combat the current pandemic. This current study exploits the natural phytochemicals obtained from clove, a traditional natural therapeutic that comprises important bioactive compounds used for targeting the main protease of SARS-CoV-2. As a result, inhibition of viral replication effectively procures by targeting the main protease, which is responsible for the viral replication inside the host. Pharmacokinetic studies were evaluated for the property of drug likeliness. A total of 53 bioactives were subjected to the study, and four among them, namely, eugenie, syzyginin B, eugenol, and Casuarictin, showed potential binding properties against the target SARS-CoV-2 main protease. The resultant best bioactive was compared with the commercially available standard drugs. Furthermore, validation of respective compounds with a comprehensive molecular dynamics simulation was performed using Schrodinger software. To further validate the bioactive phytochemicals and delimit the screening process of potential drugs against coronavirus disease 2019, in vitro and in vivo clinical studies are needed to prove their efficacy.
Unique distribution of ellagitannins in ripe strawberry fruit revealed by mass spectrometry imaging.[Pubmed:34841268]
Curr Res Food Sci. 2021 Nov 17;4:821-828.
Ellagitannins (ETs) are hydrolysable tannins composed of a polyol core, primarily glucose, which is esterified with hexahydroxydiphenic acid (HHDP), and in some cases, gallic acid. ETs are the major phenolic compounds found in strawberries and may contribute to the health-related properties of strawberries, because of their strong antioxidative activity. However, their distribution in the strawberry fruit remains unclear. In this study, matrix-assisted laser desorption/ionization-mass spectrometry imaging (MALDI-MSI) was used to visualize ETs in ripe strawberry fruits. Five peaks, corresponding to the m/z values of ET [M-H](-) ions detected in the MALDI-MS spectrum of strawberry extracts, were identified as strictinin, pedunculagin, Casuarictin, davuriicin M(1), and an unknown ET using MALDI-tandem MS (MS/MS). In addition, liquid chromatography-electrospray ionization-MS/MS of the extracts revealed the presence of pedunculagin isomers and the unknown ET. Ion images of these five ETs were reconstructed using MALDI-MSI. Strictinin was widely distributed in and around the achene seed coats, while the other ETs were dispersed in and around the seed coats, and at the bottom of the receptacle; pedunculagin was distributed in the epidermis and pith, whereas Casuarictin, the unknown ET, and davuriicin M(1) were distributed in the pith. Moreover, MALDI-MSI of a Casuarictin standard indicated that in-source fragmentation weakly affected the ion images. The results suggest that the distribution of ETs depends on the presence or absence of their constituents, namely galloyl units, HHDP, and bis-HHDP. To the best of my knowledge, this is the first report on the visualization of ETs in plant tissues using MSI, MALDI-MSI may be a useful tool for analyzing the distribution of ETs in the strawberry fruit.
Nontargeted Metabolomics as a Screening Tool for Estimating Bioactive Metabolites in the Extracts of 50 Indigenous Korean Plants.[Pubmed:34564401]
Metabolites. 2021 Aug 30;11(9):585.
Many indigenous Korean plants have been used in medicinal preparations and health-promoting foods. These plant species contain beneficial metabolites with various bioactivities, such as antioxidant and anti-inflammatory activities. Herein, we suggest a new screening strategy using metabolomics to explore the bioactive compounds in 50 Korean plants. Secondary metabolites were analyzed using UHPLC-LTQ-Orbitrap-MS/MS. The plant extracts were subjected to antioxidant and anti-inflammatory assays. We identified metabolites that contributed to bioactivities according to the results of bioassays and multivariate analyses. Using Pearson's correlation, phenolics (e.g., Casuarictin, 3-O-methylellagic acid) showed positive correlation with antioxidant activity, while biflavonoids (e.g., amentoflavone, rosbustaflavone) were correlated with nitric oxide (NO) inhibition activity. To compensate for the limitation of this new strategy, we further validated these by investigating three parts (branches, fruits, leaves) of Platycladus orientalis which showed high activities on both bioassays. Unlike the above observation, we identified significantly different metabolites from different parts, which was not the results of bioassays. In these validation steps, interestingly, biflavonoids (e.g., robustaflavone, sciadopitysin) contributed to both activities in P. orientalis. The findings of this work suggest that new strategy could be more beneficial in the identification of bioactive plant species as well as that of their corresponding bioactive compounds that impart the bioactivity.
Ellagitannin Digestion in Moth Larvae and a New Dimeric Ellagitannin from the Leaves of Platycarya strobilacea.[Pubmed:34299409]
Molecules. 2021 Jul 7;26(14):4134.
Ellagitannins (ETs) are plant polyphenols with various health benefits. Recent studies have indicated that the biological activities of ETs are attributable to their degradation products, including ellagic acid and its gut microflora metabolites, such as urolithins. Insect tea produced in the Guangxi region, China, is made from the frass of moth larvae that feed on the ET-rich leaves of Platycarya strobilacea. Chromatographic separation of the Guangxi insect tea showed that the major phenolic constituents are ellagic acid, brevifolin carboxylic acid, gallic acid, brevifolin, and polymeric polyphenols. Chemical investigation of the feed of the larvae, the fresh leaves of P. strobilacea, showed that the major polyphenols are ETs including pedunculagin, Casuarictin, strictinin, and a new ET named platycaryanin E. The new ET was confirmed as a dimer of strictinin having a tergalloyl group. The insect tea and the leaves of P. strobilacea contained polymeric polyphenols, both of which were shown to be composed of ETs and proanthocyanidins by acid hydrolysis and thiol degradation. This study clarified that Guangxi insect tea contains ET metabolites produced in the digestive tract of moth larvae, and the metabolites probably have higher bioavailabilities than the original large-molecular ETs of the leaves of P. strobilacea.
Barricyclin D1-a dimeric ellagitannin with a macrocyclic structure-and accompanying tannins from Barringtonia racemosa.[Pubmed:33890626]
Biosci Biotechnol Biochem. 2021 Jun 24;85(7):1609-1620.
Our examination of high molecular weight polyphenolic constituents in the leaves of Barringtonia racemosa of the family Lecythidaceae uncovered 5 previously undescribed ellagitannins. One, barringtin M1 (1), among them was a hydrolysable tannin monomer, while remaining 4, barringtins D1 (2), D2 (3), D3 (4), and barricyclin D1 (5), were all dimers. Barricyclin D1 had a first macrocyclic structure formed from Casuarictin (6) and tellimagrandin I (7), and the other ellagitannins had structures related to 5. Two additional known phenolics, valoneic acid dilactone (8) and schimawalin A (9), were also isolated from the leaves. These results suggested that the leaves of B. racemosa are a natural resource rich in hydrolysable tannin oligomers.
Ellagitannins from Rosa roxburghii suppress poly(I:C)-induced IL-8 production in human keratinocytes.[Pubmed:33830449]
J Nat Med. 2021 Jun;75(3):623-632.
The anti-inflammatory effects of a 50% aqueous extract of Rosa roxburghii fruit (RRFE) and two ellagitannins (strictinin and Casuarictin) isolated from the RRFE were evaluated in a cell model of skin inflammation induced by self-RNA released from epidermal cells damaged by UV ray (UVR) irradiation. The RRFE inhibited interleukin-8 (IL-8) mRNA expression in normal human epidermal keratinocytes (NHEKs) stimulated with polyinosinic:polycytidylic acid (poly(I:C)), a ligand of toll-like receptor-3 (TLR-3). The plant-derived anti-inflammatory agents, dipotassium glycyrrhizinate (GK2) and allantoin, had no influence on the IL-8 expression. The purified compounds, strictinin and Casuarictin, inhibited the IL-8 mRNA expression and IL-8 release induced in NHEKs by poly(I:C). These ellagitannins were thus found to be responsible for the biological activity exhibited by the RRFE. This study demonstrates that RRFE and isolated RRFE compounds show promise as ingredients for products formulated to improve skin disorders induced by UVR irradiation.
Casuarictin: A new herbal drug molecule for Alzheimer's disease as inhibitor of presenilin stabilization factor like protein.[Pubmed:33294689]
Heliyon. 2020 Nov 21;6(11):e05546.
Alzheimer's disease is a progressive neurodegenerative disorder. In this disease neurodegeneration occurs due to deposition of aggregated amyloid-beta plaques and neurofibrillary tangles (hyperphosphorylated tau proteins). Present study focuses on interaction of different phytochemicals with presenilin stabilization factor like protein (PSFL). PSFL protein is known to stabilize Presenilin, which is mainly involved in intramembrane hydrolysis of selected type- I membrane proteins, including amyloid-beta precursor protein, and produces amyloid-beta protein. Amyloid-beta are small peptides comprising of 36-43 amino acids, which play a significant role in senile plaques formation in the brains of Alzheimer patients. Virtual screening and docking of phytochemicals with PSFL protein was done to find the potential inhibitor. Based on binding affinity, docked energy and molecular dynamics simulations, three phytochemicals namely Saponin, Casuarictin, and Enoxolone, were identified as potential inhibitors for the target protein.
Evaluation of the Inhibitory Potential of Casuarictin, an Ellagitannin Isolated from White Mangrove (Laguncularia racemosa) Leaves, on Snake Venom Secretory Phospholipase A2.[Pubmed:31288445]
Mar Drugs. 2019 Jul 8;17(7):403.
Ellagitannins constitute the largest group of hydrolyzable tannins of plants, and, from this group, Casuarictin (Casu) was identified in some plant species. However, to our knowledge, no investigation of secretory phospholipase A2 (sPLA2) inhibition by Casu has been performed yet. Casuarictin was isolated by chromatography n-butanol (n-BuOH) partition of Laguncularia racemosa leaves. The pharmacological and biological effects of Casu were evaluated on isolated sPLA2 from the rattlesnake (Crotalus durissus terrificus) and using a plant bacterial strain. The compound was able to form a protein complex consisting of a stable sPLA2 + Casu complex. Analyses carried out with matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF) revealed that the molecular mass of sPLA2 increased from 14,425.62 to 15,362.74 Da. The enzymatic activity of the sPLA2 + Casu complex was significantly lower than that of native sPLA2. Besides, molecular interactions of Casu with sPLA2 were able to virtually abolish the native edematogenic effect as well as myonecrosis induced by the protein when injected 10 min after sPLA2. Therefore, Casu may be considered a potential anti-inflammatory that can be used to treat edema and myonecrosis induced by serine-secreting phospholipase A2. In addition, the compound also showed great antimicrobial potential.


