Tenaxin ICAS# 86926-52-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 86926-52-5 | SDF | Download SDF |
| PubChem ID | 159029.0 | Appearance | Powder |
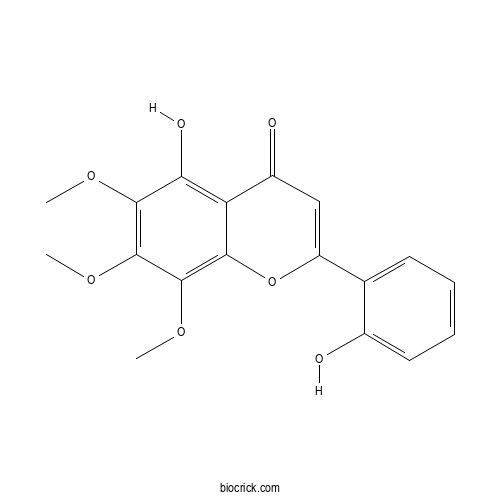
| Formula | C18H16O7 | M.Wt | 344.32 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-hydroxy-2-(2-hydroxyphenyl)-6,7,8-trimethoxychromen-4-one | ||
| SMILES | COC1=C(C(=C2C(=C1O)C(=O)C=C(O2)C3=CC=CC=C3O)OC)OC | ||
| Standard InChIKey | QCKBVAGWPBRRQJ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H16O7/c1-22-16-14(21)13-11(20)8-12(9-6-4-5-7-10(9)19)25-15(13)17(23-2)18(16)24-3/h4-8,19,21H,1-3H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Tenaxin I Dilution Calculator

Tenaxin I Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9043 mL | 14.5214 mL | 29.0428 mL | 58.0855 mL | 72.6069 mL |
| 5 mM | 0.5809 mL | 2.9043 mL | 5.8086 mL | 11.6171 mL | 14.5214 mL |
| 10 mM | 0.2904 mL | 1.4521 mL | 2.9043 mL | 5.8086 mL | 7.2607 mL |
| 50 mM | 0.0581 mL | 0.2904 mL | 0.5809 mL | 1.1617 mL | 1.4521 mL |
| 100 mM | 0.029 mL | 0.1452 mL | 0.2904 mL | 0.5809 mL | 0.7261 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ophiopogonanone D
Catalog No.:BCX1255
CAS No.:1027912-99-7
- Casuarictin
Catalog No.:BCX1254
CAS No.:79786-00-8
- Ophiopogonone C
Catalog No.:BCX1253
CAS No.:477336-77-9
- Salvadoraside
Catalog No.:BCX1252
CAS No.:143522-30-9
- Betulone
Catalog No.:BCX1251
CAS No.:7020-34-0
- D-Gluconic acid, 6-[(2E)-3-(3,4-dihydroxyphenyl)-2-propenoate]
Catalog No.:BCX1250
CAS No.:1147861-80-0
- 11(α)-Hydroxynepasaikosaponin k
Catalog No.:BCX1249
CAS No.:1152168-63-2
- 16β-Hydroperoxyalisol B 23-acetate
Catalog No.:BCX1248
CAS No.:2221029-54-3
- trans-Ferulic acid-4-β-glucoside
Catalog No.:BCX1247
CAS No.:117405-51-3
- Carmichasine B
Catalog No.:BCX1246
CAS No.:2245700-60-9
- Hypoletin-7-O-β-D-xylopyranoside
Catalog No.:BCX1245
CAS No.:126771-28-6
- Hypolaetin 7-O-glucoside
Catalog No.:BCX1244
CAS No.:32455-43-9
- Tibesaikosaponin V
Catalog No.:BCX1257
CAS No.:2319668-87-4
- Stipuleanoside R2
Catalog No.:BCX1258
CAS No.:96627-72-4
- Brevicornin
Catalog No.:BCX1259
CAS No.:173792-49-9
- cis-Pellitorine
Catalog No.:BCX1260
CAS No.:639086-18-3
- Tenacissoside A
Catalog No.:BCX1261
CAS No.:107352-30-7
- Isoasiaticoside
Catalog No.:BCX1262
CAS No.:948827-09-6
- Fallacinol
Catalog No.:BCX1263
CAS No.:569-05-1
- Tarasaponin IV
Catalog No.:BCX1264
CAS No.:156980-31-3
- Dihydrolanosterol
Catalog No.:BCX1265
CAS No.:911660-54-3
- Araloside C
Catalog No.:BCX1266
CAS No.:55446-15-6
- 3-epi-Bufalin
Catalog No.:BCX1267
CAS No.:465-20-3
- Scheffoleoside A
Catalog No.:BCX1268
CAS No.:160669-23-8
Integrated analysis of topoisomerase I inhibitors from flavonoids of Scutellaria baicalensis Georgi using bioaffinity ultrafiltration UPLC-TripleTOF MS/MS, molecular docking and target-based multiple complex networks.[Pubmed:37150329]
Fitoterapia. 2023 Jul;168:105525.
Scutellaria baicalensis Georgi (SBG) has been widely used as medical plant in East Asia with remarkable anti-cancer activity. However, the underlying mechanisms are still confused. In this study, an integrated analysis was conducted to screen topoisomerase I (topo I) inhibitors from flavonoids of SBG and investigate the anti-cancer mechanisms, containing bioaffinity ultrafiltration UPLC-ESI-TripleTOF-MS/MS, molecular docking, and multiple complex networks. The SBG extract exhibited notable cytotoxic activity on Hela cells. Five flavonoids were identified as potential topo I inhibitors, including skullcapflavone II, wogonin, chrysin, oroxylin A, and Tenaxin I. Their ESI-MS/MS spectra showed that RDA reaction and neutral molecule loss were the main fragment patterns. Docking results demonstrated that pi-pi interaction and the formation of hydrogen bond contributed most to their binding with topo I. The selected compounds, related target proteins and pathways were integrated into target-based multiple complex networks, which consisted of three subnetworks. Statistical and topological analysis of these networks revealed a series of characteristics, including scale-free property with power-law degree distribution, Poisson degree distribution, and small-world property. Chrysin, wogonin, and oroxylin A exhibited as main active components with much higher degree values. Chemical carcinogenesis-receptor activation (hsa05207) was considered as critical pathway due to remarkable centrality indexes. Additionally, potential synergistic effect of wogonin and chrysin was observed on the conversion of supercoiled DNA to relaxed forms. These results improved current understanding of flavonoid-rich plants on the treatment of cancer. Moreover, the multi-disciplinary approach provided a new strategy for the research of natural products from medical plants.
Coptis, Pinellia, and Scutellaria as a promising new drug combination for treatment of Helicobacter pylori infection.[Pubmed:36579091]
World J Clin Cases. 2022 Dec 6;10(34):12500-12514.
BACKGROUND: Helicobacter pylori (H. pylori) is the most important infectious agent and plays an important role in the progression of chronic gastritis and the development of gastric cancer. AIM: To identify efficient therapeutic agents or strategies that can treat H. pylori infection. METHODS: We performed literature analysis, experimental validation, and network pharmacology. First, traditional Chinese medicine (TCM) prescriptions for the treatment of H. pylori infection were obtained from the China National Knowledge Infrastructure, China Biology Medicine, China Science and Technology Journal Database, and WanFang databases. In addition, we conducted a relevant search by Reference Citation Analysis (RCA) (https://www.referencecitationanalysis.com). Next, we used TCM Inheritance Support System V2.5 to identify core drug combinations in the TCM prescriptions. Then, an H. pylori-associated chronic mouse model of gastritis was established. The antibacterial properties and anti-inflammatory potential of the core drug combination were evaluated by the rapid urease test, modified Warthin-Starry silver staining, histopathological analysis, and enzyme linked immunosorbent assay. Finally, the active compounds, hub targets, and potential signaling pathways associated with the core drug combination were analyzed by network pharmacology. RESULTS: The TCM treatment of H. pylori was mainly based on reinforcing the healthy Qi and eliminating pathogenic factors by simultaneously applying pungent dispersing, bitter descending, cold and warm drugs. The combination of Coptis, Pinellia, and Scutellaria (CPS) was identified as the core drug combination from 207 prescriptions and 168 herbs. This drug combination eradicated H. pylori, alleviated the gastric pathology induced by H. pylori infection, and reduced the expression levels of tumor necrosis factor-alpha (P = 0.024) and interleukin-1beta (P = 0.001). Moreover, a total of 35 compounds and 2807 targets of CPS were identified using online databases. Nine key compounds (Tenaxin I, neobaicalein, norwogonin, skullcapflavone II, baicalein, 5,8,2'-trihydroxy-7-methoxyflavone, acacetin, panicolin, and wogonin) and nine hub target proteins (EGFR, PTGS2, STAT3, MAPK3, MAPK8, HSP90AA1, MAPK1, MMP9, and MTOR) were further explored. Seventy-seven signaling pathways were correlated with H. pylori-induced inflammation and carcinogenesis. CONCLUSION: In summary, we showed that CPS is the core drug combination for treating H. pylori infection. Animal experiments demonstrated that CPS has bacteriostatic properties and can reduce the release of inflammatory cytokines in the gastric mucosa. Network pharmacology predictions further revealed that CPS showed complex chemical compositions with multi-target and multi-pathway regulatory mechanisms. Although the results derived from network pharmacology are not necessarily comprehensive, they still expand our understanding of CPS for treating H. pylori infection.
Two Novel Flavonoids with Lipid-Lowering Activity from Yi Medicine Shekaqi.[Pubmed:35934672]
Chem Biodivers. 2022 Sep;19(9):e202200363.
Yi medicine Shekaqi is the most attractive traditional ethnic medicine due to its significant and diverse pharmacological activities. Two novel flavonoids, including 5,2'-dihydroxy-6-methoxy-7-decyloxyflavone and Tenaxin II-7-O-beta-D-glucuronopyranosyl acid butyl ester, along with six known flavonoids, were isolated from Yi medicine Shekaqi. Their structures were elucidated based on the analysis of their comprehensive spectral data. The in vitro lipid-lowering activities of the eight compounds showed that all the compounds significantly inhibited the lipopolysaccharide (LPS)-induced increase in the total cholesterol (TC) level, while compounds 1, 4, 6, 7, and 8 significantly inhibited the LPS-induced increase in the triglyceride (TG) level.
Two types of O-methyltransferase are involved in biosynthesis of anticancer methoxylated 4'-deoxyflavones in Scutellaria baicalensis Georgi.[Pubmed:34490975]
Plant Biotechnol J. 2022 Jan;20(1):129-142.
The medicinal plant Scutellaria baicalensis Georgi is rich in specialized 4'-deoxyflavones, which are reported to have many health-promoting properties. We assayed Scutellaria flavones with different methoxyl groups on human cancer cell lines and found that polymethoxylated 4'-deoxyflavones, like skullcapflavone I and Tenaxin I have stronger ability to induce apoptosis compared to unmethylated baicalein, showing that methoxylation enhances bioactivity as well as the physical properties of specialized flavones, while having no side-effects on healthy cells. We investigated the formation of methoxylated flavones and found that two O-methyltransferase (OMT) families are active in the roots of S. baicalensis. The Type II OMTs, SbPFOMT2 and SbPFOMT5, decorate one of two adjacent hydroxyl groups on flavones and are responsible for methylation on the C6, 8 and 3'-hydroxyl positions, to form oroxylin A, Tenaxin II and chrysoeriol respectively. The Type I OMTs, SbFOMT3, SbFOMT5 and SbFOMT6 account mainly for C7-methoxylation of flavones, but SbFOMT5 can also methylate baicalein on its C5 and C6-hydroxyl positions. The dimethoxylated flavone, skullcapflavone I (found naturally in roots of S. baicalensis) can be produced in yeast by co-expressing SbPFOMT5 plus SbFOMT6 when the appropriately hydroxylated 4'-deoxyflavone substrates are supplied in the medium. Co-expression of SbPFOMT5 plus SbFOMT5 in yeast produced Tenaxin I, also found in Scutellaria roots. This work showed that both type I and type II OMT enzymes are involved in biosynthesis of methoxylated flavones in S. baicalensis.
Fractional analysis of dichloromethane extract of Scutellaria araxensis Grossh root and shoot by HPLC-PDA-ESI-MS(n).[Pubmed:33764217]
Nat Prod Res. 2022 Aug;36(15):4031-4035.
Scutellaria araxensis is a well-known ethnobotanical herb for the treatment of various diseases in Iranian traditional medicine. In this study, dichloromethanolic fraction of partitioned methanol root and shoot extract was analysed by silica gel column chromatography, thin layer chromatography and high-performance liquid chromatography combined with photodiode-array detector, and coupled to electrospray ionization with Q-Exactive Orbitrap mass spectrometry (HPLC-PDA-ESI-MS(n)) in positive ion mode. Metabolites were tentatively characterized by comparing their mass spectrometry spectra with those of bibliographic data. A total of 11 flavonoids were identified from different fractions. Results showed that root and shoot produced very similar flavone patterns characterized by the presence of baicalein, tricin, Tenaxin I, Tenaxin II, skullcapflavon II, chrysin, wogonin and isorhamnetin. Norwogonin and apigenin-7-glucoside were identified only from shoot. Remarkably, Tenaxin I, II, tricin, apigenin-7-glucoside and norwogonin were tentatively profiled as new compounds for the first time from S. araxensis formula.
[Studies on ethyl acetate soluble constituents of Huanglian Jiedutang].[Pubmed:19160789]
Zhongguo Zhong Yao Za Zhi. 2008 Sep;33(18):2080-6.
OBJECTIVE: To study the ethyl acetate soluble constituents from the water extractive of Huanglian Jiedutang decoction, which are composed of Rhizoma Coptidis, Radix Scutellariae, Cortex Phellodendri and Fructus Gardeniae, and provide substances foundation for its pharmacokinetic and pharmacodynamic investigation. METHOD: The chemical constituents were isolated by various column chromatographic methods and structurally elucidated by NMR and MS techniques. RESULT: Thirty-five compounds were isolated, among which twenty compounds have been identified as beta-sitosterol (1), oroxylin A (2), wogonin (3), ursolic acid (4), skullcapflavone I (5), Tenaxin I (6), skullcapflavone II (7), limonin (8), 5, 2'-dihydroxy-6, 7, 8, 3'-tetramethoxyflavone (9), chrysin (12), baicalein (17), Tenaxin II (19), 5, 7, 2'-trihydroxy-6, 8-dimethoxyflavone (21), shihulimonin A (22), 6, 2'-dihydroxy-5, 7, 8, 6'-tetramethoxyflavone (26), viscidulin II (28), 5, 7, 4'-trihydroxy-8-methoxyflavone (29), 5, 7, 2', 6'-tetrahydroxyflavone (30), wogonin-7-O-beta-D-glucuronide methyl ester (31) and daucosterol (34). CONCLUSION: On the basis of reported results of the chemical constituents of Rhizoma Coptidis, Radix Scutellariae, Cortex Phellodendri and Fructus Gardeniae, it was estimated that all flavonoid compounds rised from the Radix Scutellariae, and compounds 8 and 22 rised from Cortex Phellodendri. Compound 22 was identified in the Cortex Phellodendri for the first time.


