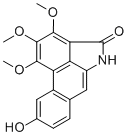
StigmalactamCAS# 289499-72-5 |
Quality Control & MSDS
Package In Stock
Number of papers citing our products

| Cas No. | 289499-72-5 | SDF | File under preparation. |
| PubChem ID | N/A | Appearance | Yellow powder |
| Formula | C18H15NO5 | M.Wt | 325.3 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Stigmalactam Dilution Calculator

Stigmalactam Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0741 mL | 15.3704 mL | 30.7409 mL | 61.4817 mL | 76.8521 mL |
| 5 mM | 0.6148 mL | 3.0741 mL | 6.1482 mL | 12.2963 mL | 15.3704 mL |
| 10 mM | 0.3074 mL | 1.537 mL | 3.0741 mL | 6.1482 mL | 7.6852 mL |
| 50 mM | 0.0615 mL | 0.3074 mL | 0.6148 mL | 1.2296 mL | 1.537 mL |
| 100 mM | 0.0307 mL | 0.1537 mL | 0.3074 mL | 0.6148 mL | 0.7685 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isorengyol
Catalog No.:BCN0481
CAS No.:101489-38-7
- 4-(Acetoxymethyl)phenyl glucoside
Catalog No.:BCN0480
CAS No.:188290-72-4
- Kokusaginine
Catalog No.:BCN0479
CAS No.:484-08-2
- 2,4,6-Trihydroxy-3-prenylacetophenone
Catalog No.:BCN0478
CAS No.:27364-71-2
- Ferruginoside C
Catalog No.:BCN0477
CAS No.:213991-03-8
- (±)-8-Gingerol
Catalog No.:BCN0476
CAS No.:77398-92-6
- 2',3'-Dehydromarmesin
Catalog No.:BCN0475
CAS No.:28664-60-0
- Polypodine B 20,22-acetonide
Catalog No.:BCN0474
CAS No.:159858-85-2
- Acrotrione
Catalog No.:BCN0473
CAS No.:2349327-31-5
- 1,4-Dihydro-1-methyl-4-oxonicotinamide
Catalog No.:BCN0472
CAS No.:769-49-3
- 1-O-Cinnamoylglucose
Catalog No.:BCN0471
CAS No.:40004-96-4
- 1-O-p-Coumaroylglucose
Catalog No.:BCN0470
CAS No.:7139-64-2
- [6]-Gingerol
Catalog No.:BCN0483
CAS No.:39886-76-5
- Acrovestone
Catalog No.:BCN0484
CAS No.:24177-16-0
- Marionol
Catalog No.:BCN0485
CAS No.:65602-55-3
- Verproside
Catalog No.:BCN0486
CAS No.:50932-20-2
- 5-Hydroxy-6,7,3',4'-tetramethoxyflavone
Catalog No.:BCN0487
CAS No.:21763-80-4
- Piperdardine
Catalog No.:BCN0488
CAS No.:188426-70-2
- 4''-O-Methylcatalposide
Catalog No.:BCN0489
CAS No.:887140-17-2
- Apigenin 7,4'-di-O-alloside
Catalog No.:BCN0490
CAS No.:95693-63-3
- Tsaokoin
Catalog No.:BCN0491
CAS No.:343605-41-4
- Piperchabamide B
Catalog No.:BCN0492
CAS No.:807618-21-9
- Piceatannol 4'-O-glucoside
Catalog No.:BCN0493
CAS No.:116181-54-5
- 6-O-Veratroylcatalpol
Catalog No.:BCN0494
CAS No.:56973-43-4
Chemical constituents and antibacterial activity from the stems and leaves of Piper wallichii.[Pubmed:34085561]
J Asian Nat Prod Res. 2021 Jun 4:1-8.
A phytochemical investigation of the stems and leaves of Piper wallichii led to the isolation of two new compounds, an aryl alkanone, piwalkanone (1) and a dioxoaporphine alkaloid, piwallidione (2), together with nine known compounds, a dioxoaporphine alkaloid, cepharadione A (3); two aristolactams, piperolactam A (4) and Stigmalactam (5); a piperidine, piperine (6); four isobutylamides, piperlonguminine (7), pellitorine (8), N-isobutyl-2E,4E-octadecadienamide (9), and guineensine (10); and a tyramine, N-trans-feruloyltyramine (11). Their structures were elucidated on the basis of spectroscopic evidence (IR, (1)H NMR, (13)C NMR and 2 D NMR) and MS. Compounds 2 and 3 showed inhibitory activities against pathogenic bacteria, Bacillus cereus, Bacillus subtilis and Staphylococcus aureus.
Stigmalactam from Orophea enterocarpa induces human cancer cell apoptosis via a mitochondrial pathway.[Pubmed:25556482]
Asian Pac J Cancer Prev. 2014;15(23):10397-400.
Stigmalactam, an aristolactam-type alkaloid extracted from Orophea enterocarpa, exerts cytotoxicity against several human and murine cancer cell lines, but the molecular mechanisms remain elusive. The aims of this study were to identify the mode and mechanisms of human cancer cell death induced by Stigmalactam employing human hepatocellular carcinoma HepG2 and human invasive breast cancer MDA-MB-231 cells as models, compared to normal murine fibroblasts. It was found that Stigmalactam was toxic to HepG2 and MDA-MB-231 cells with IC50 levels of 23.0+/-2.67 muM and 33.2+/-4.54 muM, respectively, using MTT assays. At the same time the IC50 level towards murine normal fibroblast NIH3T3 cells was 24.4+/-6.75 muM. Reactive oxygen species (ROS) production was reduced in Stigmalactam-treated cells dose dependently after 4 h of incubation, indicating antioxidant activity, measured by using 2',7',-dichlorohydrofluorescein diacetate and flow cytometry. Caspase-3 and caspase-9 activities were increased in a dose response manner, while Stigmalactam decreased the mitochondrial transmembrane potential dose-dependently in HepG2 cells, using 3,3'-dihexyloxacarbocyanine iodide and flow cytometry, indicating mitochondrial pathway-mediated apoptosis. In conclusion, Stigmalactam from O. enterocarpa was toxic to both HepG2 and MDA-MB-231 cells and induced human cancer HepG2 cells to undergo apoptosis via the intrinsic (mitochondrial) pathway.
Aristolactam-type alkaloids from Orophea enterocarpa and their cytotoxicities.[Pubmed:22606026]
Int J Mol Sci. 2012;13(4):5010-8.
A new aristolactam, named enterocarpam-III (10-amino-2,3,4,6-tetramethoxy phenanthrene-1-carboxylic acid lactam, 1) together with the known alkaloid Stigmalactam (2), were isolated from Orophea enterocarpa. Their structures were elucidated on the basis of interpretation of their spectroscopic data. Compounds 1 and 2 exhibited significant cytotoxicities against human colon adenocarcinoma (HCT15) cell line with IC(50) values of 1.68 and 1.32 muM, respectively.
Aristolactams from roots of Ottonia anisum (Piperaceae).[Pubmed:21834228]
Nat Prod Commun. 2011 Jul;6(7):939-42.
The Piperaceae species are known worldwide for its medicinal properties and its chemical compounds. In Brazil, many species of this family are distributed mainly in Amazon Region and in the Atlantic Forest. The genus Ottonia is known as source of amides, flavonoids, arilpropanoids and terpenes with record biological activities. Six aristolactams, including, aristolactam BII, piperolactam C, goniothalactam, Stigmalactam, aristolactam AII and aristolactam BIII were isolated from roots of this species. GC-MS, 1H NMR and NOESY techniques were used to characterize these compounds. This is the first report about the occurrence of aristolactams in the Ottonia anisum Sprengel.
Aristolactams and dioxoaporphines from Fissistigma balansae and Fissistigma oldhamii.[Pubmed:10978218]
J Nat Prod. 2000 Aug;63(8):1160-3.
Investigation of extracts of Fissistigma balansae and Fissistigma oldhamii resulted in the isolation of 11 aristolactams-Stigmalactam (1), piperolactam A (2), piperolactam C (3), aristolactam AII (4), aristolactam AIIIa (5), aristolactam BII (6), aristolactam BIII (7), aristolactam FII (8), goniothalactam (9), enterocarpam I (10), and velutinam (11)-as well as two dioxoaporphines, noraristolodione (12) and norcepharadione B (13). The new compound 1 was identified by spectral data interpretation. Compounds 1-13 were subjected to antiplatelet aggregation testing.


