TariquidarP-glycoprotein inhibitor,potent and non-competitive CAS# 206873-63-4 |
- Valspodar
Catalog No.:BCC2027
CAS No.:121584-18-7
- Dofequidar
Catalog No.:BCC4176
CAS No.:129716-58-1
- Elacridar
Catalog No.:BCC1546
CAS No.:143664-11-3
- Elacridar hydrochloride
Catalog No.:BCC1547
CAS No.:143851-98-3
- LY335979 (Zosuquidar 3HCL)
Catalog No.:BCC3878
CAS No.:167465-36-3
- Tariquidar methanesulfonate, hydrate
Catalog No.:BCC1986
CAS No.:625375-83-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 206873-63-4 | SDF | Download SDF |
| PubChem ID | 148201 | Appearance | Powder |
| Formula | C38H38N4O6 | M.Wt | 646.73 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | XR9576 | ||
| Solubility | DMSO : 2.5 mg/mL (3.87 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
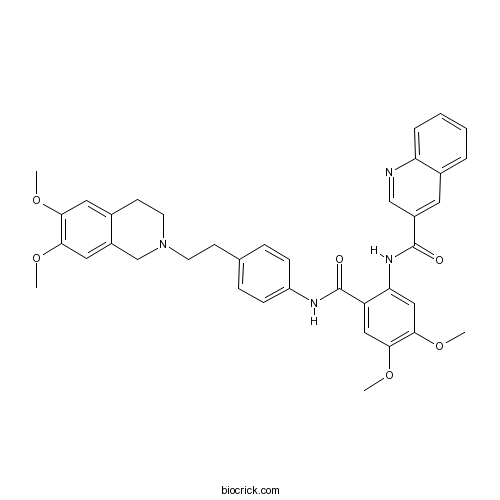
| Chemical Name | N-[2-[[4-[2-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)ethyl]phenyl]carbamoyl]-4,5-dimethoxyphenyl]quinoline-3-carboxamide | ||
| SMILES | COC1=C(C=C2CN(CCC2=C1)CCC3=CC=C(C=C3)NC(=O)C4=CC(=C(C=C4NC(=O)C5=CC6=CC=CC=C6N=C5)OC)OC)OC | ||
| Standard InChIKey | LGGHDPFKSSRQNS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C38H38N4O6/c1-45-33-18-25-14-16-42(23-28(25)19-34(33)46-2)15-13-24-9-11-29(12-10-24)40-38(44)30-20-35(47-3)36(48-4)21-32(30)41-37(43)27-17-26-7-5-6-8-31(26)39-22-27/h5-12,17-22H,13-16,23H2,1-4H3,(H,40,44)(H,41,43) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tariquidar (XR9576) is a potent and selective noncompetitive inhibitor of P-glycoprotein with Kd of 5.1 nM. | |||||
| Targets | P-glycoprotein | |||||
| IC50 | 5.1 nM (Kd) | |||||
| Cell experiment: [1] | |
| Cell lines | KB-3-1, KB-8-5-11 (ABCB1-expressing variant), MCF-7, MCF-7/VP16 (ABCC1-expressing variant), H460, H460/MX20 (ABCG2-expressing variant) |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | ≥ 100 nM |
| Applications | At concentrations ≥ 100 nM, tariquidar inhibited both P-gp and BCRP but did not inhibit MRP1. Accumulation of the fluorescent substrate calcein-AM in ABCB1-expressing cells treated with 100 nM and 1 μM tariquidar increased 14-fold and 19-fold, respectively. Most P-gp was inhibited at 100 nM. At the same concentrations, tariquidar also increased the accumulation of the fluorescent substrate mitoxantrone in ABCG2-expressing cells by 4-fold (P<0.001) and 8-fold (P<0.001), respectively. These data indicate that tariquidar inhibits both transporters with similar potency because at 100 nM, it restored accumulation to 56% of control for P-gp and 84% of control for BCRP. Tariquidar did not increase accumulation of substrate in ABCC1-expressing cells. |
| Animal experiment: [2] | |
| Animal models | NMRI nu/nu mice |
| Dosage form | Oral administration, 0.1 ml/10 g of body weight |
| Application | The ABCB1 modulator tariquidar affects the distribution of paclitaxel in nude mice. In the brains, Co-application of tariquidar with paclitaxel led to a comparable increase in the brain concentration of the cytostatic by a factor of 2.5-to 6.7. In liver, no statistically significant differences were determined between the different ABCB1 modulator group and the control group. In the kidneys, the paclitaxel content in kidney decreased to achieve concentrations similar to those in the untreated control group. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Kannan P, Telu S, Shukla S, et al. The “specific” P-glycoprotein inhibitor tariquidar is also a substrate and an inhibitor for breast cancer resistance protein (BCRP/ABCG2). ACS chemical neuroscience, 2010, 2(2): 82-89. [2] Hubensack M, Müller C, Höcherl P, et al. Effect of the ABCB1 modulators elacridar and tariquidar on the distribution of paclitaxel in nude mice. Journal of cancer research and clinical oncology, 2008, 134(5): 597-607. | |

Tariquidar Dilution Calculator

Tariquidar Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.5462 mL | 7.7312 mL | 15.4624 mL | 30.9248 mL | 38.656 mL |
| 5 mM | 0.3092 mL | 1.5462 mL | 3.0925 mL | 6.185 mL | 7.7312 mL |
| 10 mM | 0.1546 mL | 0.7731 mL | 1.5462 mL | 3.0925 mL | 3.8656 mL |
| 50 mM | 0.0309 mL | 0.1546 mL | 0.3092 mL | 0.6185 mL | 0.7731 mL |
| 100 mM | 0.0155 mL | 0.0773 mL | 0.1546 mL | 0.3092 mL | 0.3866 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Tariquidar is a potent inhibitor of P-glycoprotein (Pgp), a 170-kDa transmembrane protein acting as a drug efflux pump to actively transport structurally unrelated compounds out of cells, that noncompetitively inhibits the basal the activity of ATPase associated with Pgp. Tariquidar contains a tertiary amine, dimethoxyphenyl group and amide group in its chemical structure, which contribute to its inhibition against Pgp. Results of in vitro assays of three different models have shown that Tariquidar inhibits Pgp with 50% inhibition concentration IC50 values ranging from 15 to 223 nM. However, the inhibition by tariquidar is Pgp-specific and attenuated in tumor cell lines, where multidrug resistance is mediated by multidrug resistance-associated protein.
Reference
Fox E, Bates SE. Tariquidar (XR9576): a P-glycoprotein drug efflux pump inhibitor. Expert Rev Anticancer Ther. 2007 Apr;7(4):447-59.
Bankstahl JP, Bankstahl M, Römermann K, Wanek T, Stanek J, Windhorst AD, Fedrowitz M, Erker T, Müller M, Löscher W, Langer O, Kuntner C. Tariquidar and elacridar are dose-dependently transported by P-glycoprotein and Bcrp at the blood-brain barrier: a small-animal positron emission tomography and in vitro study. Drug Metab Dispos. 2013 Apr;41(4):754-62. doi: 10.1124/dmd.112.049148. Epub 2013 Jan 10.
- Vincristine sulfate
Catalog No.:BCN2542
CAS No.:2068-78-2
- 3,7-Di-O-methylquercetin
Catalog No.:BCN6486
CAS No.:2068-02-2
- Pedatisectine F
Catalog No.:BCN4902
CAS No.:206757-32-6
- Ecliptasaponin D
Catalog No.:BCN2760
CAS No.:206756-04-9
- Cannabichromene
Catalog No.:BCN4901
CAS No.:20675-51-8
- Pasiniazid
Catalog No.:BCC3835
CAS No.:2066-89-9
- 7-O-Methylporiol
Catalog No.:BCN3948
CAS No.:206560-99-8
- Carmichaenine E
Catalog No.:BCN7730
CAS No.:2065228-63-7
- Carmichaenine D
Catalog No.:BCN7732
CAS No.:2065228-62-6
- Carmichaenine C
Catalog No.:BCN7731
CAS No.:2065228-61-5
- Carmichaenine B
Catalog No.:BCN7733
CAS No.:2065228-60-4
- Carmichaenine A
Catalog No.:BCN7729
CAS No.:2065228-59-1
- Methysticin
Catalog No.:BCN2306
CAS No.:20697-20-5
- BD 1063 dihydrochloride
Catalog No.:BCC6832
CAS No.:206996-13-6
- Neosperidin dihydrochalcone
Catalog No.:BCN5016
CAS No.:20702-77-6
- Norlichexanthone
Catalog No.:BCN4903
CAS No.:20716-98-7
- Fustin
Catalog No.:BCN4904
CAS No.:20725-03-5
- TB 21007
Catalog No.:BCC7510
CAS No.:207306-50-1
- Methyl sinapate
Catalog No.:BCN4675
CAS No.:20733-94-2
- 1,8-Bis(dimethylamino)naphtalene
Catalog No.:BCC8429
CAS No.:20734-58-1
- Saikosaponin C
Catalog No.:BCN1087
CAS No.:20736-08-7
- Saikosaponin A
Catalog No.:BCN1086
CAS No.:20736-09-8
- Dihydrosinapylalcohol
Catalog No.:BCN6542
CAS No.:20736-25-8
- 3-Bromocytisine
Catalog No.:BCC7705
CAS No.:207390-14-5
The reversal of multidrug resistance in ovarian carcinoma cells by co-application of tariquidar and paclitaxel in transferrin-targeted polymeric micelles.[Pubmed:27616277]
J Drug Target. 2017 Mar;25(3):225-234.
In this study, a transferrin (Tf)-modified polyethylene glycol-phosphatidyl ethanolamine (PEG-PE)-based micellar delivery system containing paclitaxel (PTX) and Tariquidar (TRQ), a potent third generation P-gp inhibitor, was prepared. The nanoformulation was evaluated by targeting efficiency, cellular association, cellular internalization pathway and cytotoxicity for reversal of PTX resistance on two multidrug resistant (MDR) ovarian carcinoma cell lines, SKOV-3TR and A2780-Adr. PTX and TRQ are both hydrophobic compounds. They were successfully encapsulated into the micellar structure containing vitamin E as the encapsulation enhancer. The Tf-targeted micelles were internalized mainly via clathrin-dependent endocytosis by both cell lines. For SKOV-3TR, additional mechanisms including caveolin-dependent endocytosis and macropinocytosis were found to play a significant role. The PTX cytotoxicity against the SKOV-3TR and A2780-Adr MDR cells was increased significantly in the presence of micellar encapsulation. However, unlike the A2780-Adr cell line, the Tf-targeting effect was significant on SKOV-3TR cells when co-administrated with TRQ. Penetration of the Tf-targeted micelles in a cancer cell spheroid culture was also investigated.
Whole-Body Distribution and Radiation Dosimetry of 11C-Elacridar and 11C-Tariquidar in Humans.[Pubmed:27081167]
J Nucl Med. 2016 Aug;57(8):1265-8.
UNLABELLED: (11)C-elacridar and (11)C-Tariquidar are new PET tracers to assess the transport activity of P-glycoprotein (adenosine triphosphate-binding cassette subfamily B, member 1 [ABCB1]) and breast cancer resistance protein (adenosine triphosphate-binding cassette subfamily G, member 2 [ABCG2]). This study investigated the whole-body distribution and radiation dosimetry of both radiotracers in humans. METHODS: Twelve healthy volunteers (6 women, 6 men) underwent whole-body PET/CT imaging over the 90 min after injection of either (11)C-elacridar or (11)C-Tariquidar. Radiation doses were calculated with OLINDA/EXM software using adult reference phantoms. RESULTS: Biodistribution was consistent with a major elimination route of hepatobiliary excretion, which may be mediated by ABCB1 and ABCG2. High radioactivity uptake was seen in liver, followed by spleen and kidneys, whereas brain uptake was lowest. Effective doses were 3.41 +/- 0.06 muSv/MBq for (11)C-elacidar and 3.62 +/- 0.11 muSv/MBq for (11)C-Tariquidar. CONCLUSION: Our data indicate that both (11)C-elacridar and (11)C-Tariquidar are safe radiotracers, for which an injected activity of 400 MBq corresponds to a total effective dose of approximately 1.5 mSv.
Preparation of multilocation reduction-sensitive core crosslinked folate-PEG-coated micelles for rapid release of doxorubicin and tariquidar to overcome drug resistance.[Pubmed:28055982]
Nanotechnology. 2017 Feb 24;28(8):085603.
Herein, we prepared folate-targeting core crosslinked polymeric micelles (CCL/FA) containing multiple disulfide bonds located at the interface and core of the micelles to co-deliver doxorubicin (DOX) and the P-glycoprotein (P-gp) inhibitor Tariquidar (TQR) for reversing drug resistance. The stability and redox-responsive behavior of the CCL/FA micelles was evaluated through the changes in morphology, molecular weight and hydrodynamic size. On the one hand, the micelles possessed good stability, which led to the suppression of drug release from the CCL micelles in the physiological environment. On the other hand, under reductive conditions, the CCL micelles collapsed rapidly and accelerated drug release markedly. In vitro cytotoxicity measurements, combined with confocal laser scanning microscopy (CLSM) and flow cytometry, confirmed that the dual-drug-loaded micelles exhibited obviously higher cytotoxicity to MCF-7/ADR-resistant cells than free DOX . HCl, single-drug loaded CCL micelles and nontargeted CCL micelles. The results imply that co-delivering DOX and TQR by CCL/FA micelles may be a promising way of overcoming multidrug resistance in tumor treatments.


