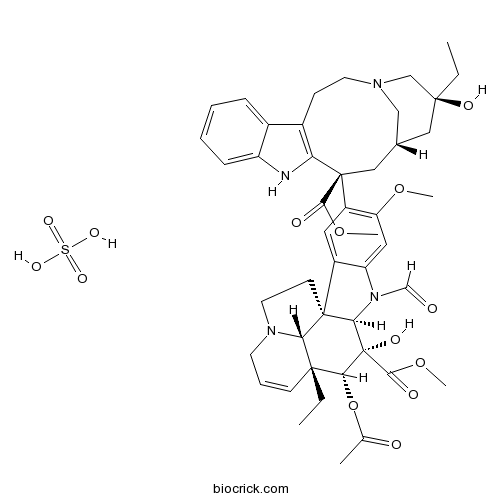
Vincristine sulfateMicrotubule disrupter,antitumor agent CAS# 2068-78-2 |
- Docetaxel
Catalog No.:BCN5342
CAS No.:114977-28-5
- ABT-751 (E7010)
Catalog No.:BCC1085
CAS No.:141430-65-1
- Epothilone A
Catalog No.:BCC1091
CAS No.:152044-53-6
- Epothilone B (EPO906, Patupilone)
Catalog No.:BCC1092
CAS No.:152044-54-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2068-78-2 | SDF | Download SDF |
| PubChem ID | 249332 | Appearance | Powder |
| Formula | C46H58N4O14S | M.Wt | 923.04 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Leurocristine sulfate; 22-Oxovincaleukoblastine sulfate | ||
| Solubility | DMSO : 82.5 mg/mL (89.38 mM; Need ultrasonic) | ||
| SMILES | CCC1(CC2CC(C3=C(CCN(C2)C1)C4=CC=CC=C4N3)(C5=C(C=C6C(=C5)C78CCN9C7C(C=CC9)(C(C(C8N6C=O)(C(=O)OC)O)OC(=O)C)CC)OC)C(=O)OC)O.OS(=O)(=O)O | ||
| Standard InChIKey | AQTQHPDCURKLKT-JKDPCDLQSA-N | ||
| Standard InChI | InChI=1S/C46H56N4O10.H2O4S/c1-7-42(55)22-28-23-45(40(53)58-5,36-30(14-18-48(24-28)25-42)29-12-9-10-13-33(29)47-36)32-20-31-34(21-35(32)57-4)50(26-51)38-44(31)16-19-49-17-11-15-43(8-2,37(44)49)39(60-27(3)52)46(38,56)41(54)59-6;1-5(2,3)4/h9-13,15,20-21,26,28,37-39,47,55-56H,7-8,14,16-19,22-25H2,1-6H3;(H2,1,2,3,4)/t28-,37+,38-,39-,42+,43-,44-,45+,46+;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Vincristine sulfate is an antitumor vinca alkaloid which inhibits microtubule formation in mitotic spindle, resulting in an arrest of dividing cells at the metaphase stage. It binds to microtubule with a Ki of 85 nM. |
| In vitro | Delivery of vincristine sulfate-conjugated gold nanoparticles using liposomes: a light-responsive nanocarrier with enhanced antitumor efficiency.[Pubmed: 25960649]Int J Nanomedicine. 2015 Apr 22;10:3081-95.The present study explored the use of drug-gold nanoparticle conjugates incorporated into liposomes to enhance antitumor efficiency. A model drug, Vincristine sulfate, was physically conjugated with gold nanoparticles and verified by UV-visible and fourier transform infrared spectroscopy, and differential scanning calorimetry. Erythrocyte dysplasia in peripheral blood smears from 5 thrombocytopenic dogs treated with vincristine sulfate.[Pubmed: 24138476]Vet Clin Pathol. 2013 Dec;42(4):458-64.Secondary dyserythropoiesis has been associated with vincristine administration in dogs. Evaluation of bone marrow aspirates for the presence of morphologic abnormalities in the erythroid lineage aids in the diagnosis. However, morphologic features of circulating erythroid precursors in these cases have not been described previously. The purpose of this report was to describe the cytologic features of dyserythropoiesis in peripheral blood and also bone marrow smears in a case series of dogs with immune-mediated thrombocytopenia (IMT) treated with Vincristine sulfate. |
| In vivo | High-dose vincristine sulfate liposome injection (Marqibo) Is not associated with clinically meaningful hematologic toxicity.[Pubmed: 24417913]Clin Lymphoma Myeloma Leuk. 2014 Jun;14(3):197-202.Vincristine sulfate liposome injection at its approved dose resulted in a low incidence of clinically meaningful hematologic toxicity. A near doubling of the median dose density did not have an identifiable effect on the reported incidence and severity of hematologic AEs. Vincristine sulfate liposome injection could be well suited for use combined with myelosuppresive drugs and for patients unable to tolerate peripheral blood cytopenia. |
| Structure Identification | J Drug Target. 2014 Jul;22(6):509-17.Preparation of vincristine sulfate-loaded poly (butylcyanoacrylate) nanoparticles modified with pluronic F127 and evaluation of their lymphatic tissue targeting.[Pubmed: 24625287]In order to improve the lymphatic targeting efficiency of anti-cancer agent Vincristine sulfate (VCR), the poly (butylcyanoacrylate) nanoparticles (VCR-PBCA-NPs) were prepared by emulsion polymerization and modified superficially with Pluronic F127. |

Vincristine sulfate Dilution Calculator

Vincristine sulfate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.0834 mL | 5.4169 mL | 10.8338 mL | 21.6675 mL | 27.0844 mL |
| 5 mM | 0.2167 mL | 1.0834 mL | 2.1668 mL | 4.3335 mL | 5.4169 mL |
| 10 mM | 0.1083 mL | 0.5417 mL | 1.0834 mL | 2.1668 mL | 2.7084 mL |
| 50 mM | 0.0217 mL | 0.1083 mL | 0.2167 mL | 0.4334 mL | 0.5417 mL |
| 100 mM | 0.0108 mL | 0.0542 mL | 0.1083 mL | 0.2167 mL | 0.2708 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Vincristine is a naturally occurring alkaloid that potently inhibits the tubulin addition with the inhibition constant Ki value of 0.085 μM and exhibits an anti-proliferative activity against B16 melanoma cells with the half maximal inhibition concentration IC50 value of 0.45 M [1].
Vincristine is extracted from leaves of the periwinkle plant Catharanthus roseus (L.) G. Don of the family Apocynaceae and has an asymmetric structure consisting of two dimers, a dihydroindole nucleus (vindoline) and an indole nucleus (catharanthine), that are linked by a carbon-carbon bond [2].
Vincristine has been found to be active against a wild range of malignancies, including acute lymphoblastic leukemia (ALL), acute non-lymphoblastic leukemia (ANLL), non-Hodgkin lymphoma (NHL), Hodgkin’s disease and brain tumors [2].
References:
[1] Jordan MA, Himes RH, Wilson L. Comparison of the effects of vinblastine, vincristine, vindesine, and vinepidine on microtubule dynamics and cell proliferation in vitro. Cancer Res. 1985 Jun;45(6):2741-7.
[2] Gidding CE1, Kellie SJ, Kamps WA, de Graaf SS. Vincristine revisited. Crit Rev Oncol Hematol. 1999 Feb;29(3):267-87.
- 3,7-Di-O-methylquercetin
Catalog No.:BCN6486
CAS No.:2068-02-2
- Pedatisectine F
Catalog No.:BCN4902
CAS No.:206757-32-6
- Ecliptasaponin D
Catalog No.:BCN2760
CAS No.:206756-04-9
- Cannabichromene
Catalog No.:BCN4901
CAS No.:20675-51-8
- Pasiniazid
Catalog No.:BCC3835
CAS No.:2066-89-9
- 7-O-Methylporiol
Catalog No.:BCN3948
CAS No.:206560-99-8
- Carmichaenine E
Catalog No.:BCN7730
CAS No.:2065228-63-7
- Carmichaenine D
Catalog No.:BCN7732
CAS No.:2065228-62-6
- Carmichaenine C
Catalog No.:BCN7731
CAS No.:2065228-61-5
- Carmichaenine B
Catalog No.:BCN7733
CAS No.:2065228-60-4
- Carmichaenine A
Catalog No.:BCN7729
CAS No.:2065228-59-1
- Sinapaldehyde
Catalog No.:BCN4900
CAS No.:20649-43-8
- Tariquidar
Catalog No.:BCC3625
CAS No.:206873-63-4
- Methysticin
Catalog No.:BCN2306
CAS No.:20697-20-5
- BD 1063 dihydrochloride
Catalog No.:BCC6832
CAS No.:206996-13-6
- Neosperidin dihydrochalcone
Catalog No.:BCN5016
CAS No.:20702-77-6
- Norlichexanthone
Catalog No.:BCN4903
CAS No.:20716-98-7
- Fustin
Catalog No.:BCN4904
CAS No.:20725-03-5
- TB 21007
Catalog No.:BCC7510
CAS No.:207306-50-1
- Methyl sinapate
Catalog No.:BCN4675
CAS No.:20733-94-2
- 1,8-Bis(dimethylamino)naphtalene
Catalog No.:BCC8429
CAS No.:20734-58-1
- Saikosaponin C
Catalog No.:BCN1087
CAS No.:20736-08-7
- Saikosaponin A
Catalog No.:BCN1086
CAS No.:20736-09-8
- Dihydrosinapylalcohol
Catalog No.:BCN6542
CAS No.:20736-25-8
High-dose vincristine sulfate liposome injection (Marqibo) Is not associated with clinically meaningful hematologic toxicity.[Pubmed:24417913]
Clin Lymphoma Myeloma Leuk. 2014 Jun;14(3):197-202.
BACKGROUND: Vincristine sulfate liposome injection (VSLI) facilitates vincristine dose intensification and densification, is active in untreated and relapsed lymphoma, and has been approved in the United States for relapsed and refractory acute lymphoblastic leukemia. Cancer- and concomitant chemotherapy-related anemia, neutropenia, and thrombocytopenia in patients with hematologic malignancy have complicated the evaluation of hematologic toxicity related to new drugs. PATIENTS AND METHODS: We assessed the hematologic toxicity of VSLI 2.25 mg/m(2) administered every 14 (cohort 1) or 7 (cohort 2) days in 54 patients with metastatic uveal melanoma, a cancer not known to involve the bone marrow. RESULTS: Cohort 2 received a greater median number of VSLI doses (6 vs. 4) within a shorter median period (5.7 vs. 8.7 weeks), resulting in a larger median cumulative exposure (22.6 vs. 17.7 mg) and near doubling of the median dose density (2.2 vs. 4.0 mg/wk) compared with cohort 1. Despite greater VSLI exposure and dose density, cohort 2 had a lower median decrease from baseline in the neutrophil count and a greater increase from baseline in the platelet count compared with cohort 1. Hematologic adverse events (AEs) were uncommon and mostly grade 1 or 2 in severity. No grade 4 hematologic AEs developed. CONCLUSION: VSLI at its approved dose resulted in a low incidence of clinically meaningful hematologic toxicity. A near doubling of the median dose density did not have an identifiable effect on the reported incidence and severity of hematologic AEs. VSLI could be well suited for use combined with myelosuppresive drugs and for patients unable to tolerate peripheral blood cytopenia.
Erythrocyte dysplasia in peripheral blood smears from 5 thrombocytopenic dogs treated with vincristine sulfate.[Pubmed:24138476]
Vet Clin Pathol. 2013 Dec;42(4):458-64.
Secondary dyserythropoiesis has been associated with vincristine administration in dogs. Evaluation of bone marrow aspirates for the presence of morphologic abnormalities in the erythroid lineage aids in the diagnosis. However, morphologic features of circulating erythroid precursors in these cases have not been described previously. The purpose of this report was to describe the cytologic features of dyserythropoiesis in peripheral blood and also bone marrow smears in a case series of dogs with immune-mediated thrombocytopenia (IMT) treated with Vincristine sulfate. Nineteen dogs receiving vincristine for treatment of IMT were identified by retrospectively searching a computerized medical record system. There were 5 dogs that had dysplastic erythroid precursors in peripheral blood smears within 7 days of vincristine treatment. Two of those 5 dogs also had evidence for erythrodysplasia in modified Wright's-stained bone marrow smears obtained postvincristine administration. Morphologic changes included bizarre or inappropriate mitotic figures, abnormal nuclear configurations (fragmentation, elongation, indentation, and binucleation), atypical nuclear remnants (Howell-Jolly bodies), or nuclear and cytoplasmic asynchrony within the erythroid precursors. A brief review of the literature with discussion of the etiologies for dyserythropoiesis is provided. The dyserythropoiesis was clinically insignificant in all 5 cases and resolved. However, pathologists and clinicians should be aware of these potential findings to prevent misdiagnosis of other conditions.
Preparation of vincristine sulfate-loaded poly (butylcyanoacrylate) nanoparticles modified with pluronic F127 and evaluation of their lymphatic tissue targeting.[Pubmed:24625287]
J Drug Target. 2014 Jul;22(6):509-17.
In order to improve the lymphatic targeting efficiency of anti-cancer agent Vincristine sulfate (VCR), the poly (butylcyanoacrylate) nanoparticles (VCR-PBCA-NPs) were prepared by emulsion polymerization and modified superficially with Pluronic F127. These prepared nanoparticles with (F127-VCR-PBCA-NPs) and without surface modification (VCR-PBCA-NPs) were characterized and their lymphatic targeting efficiencies were evaluated in vitro and in vivo. The results showed that VCR was released more sustained from both kinds of VCR-loaded nanoparticles, compared with the VCR solution. The up-taking efficiency of VCR into raji cells was enhanced by F127-VCR-PBCA-NPs, compared with the VCR-PBCA-NPs or VCR solution. Lower clearance (CL) of VCR from the systemic circulation and higher lymphatic targeting efficiency of VCR were observed for F127-VCR-PBCA-NPs than the VCR-PBCA-NPs or VCR solution, and F127-VCR-PBCA-NPs showed greater antitumor efficacy than the VCR-PBCA-NPs or VCR solution in the human Burkitt's lymphoma (raji)-bearing nude mice. These findings suggest that superficially modified nanoscale carriers might be promising vehicles for chemotherapeutic agents in the treatments of metastatic tumors and malignant lymphoma.
Delivery of vincristine sulfate-conjugated gold nanoparticles using liposomes: a light-responsive nanocarrier with enhanced antitumor efficiency.[Pubmed:25960649]
Int J Nanomedicine. 2015 Apr 22;10:3081-95.
Rapid drug release at the specific site of action is still a challenge for antitumor therapy. Development of stimuli-responsive hybrid nanocarriers provides a promising strategy to enhance therapeutic effects by combining the unique features of each component. The present study explored the use of drug-gold nanoparticle conjugates incorporated into liposomes to enhance antitumor efficiency. A model drug, Vincristine sulfate, was physically conjugated with gold nanoparticles and verified by UV-visible and fourier transform infrared spectroscopy, and differential scanning calorimetry. The conjugates were incorporated into liposomes by film dispersion to yield nanoparticles (113.4 nm) with light-responsive release properties, as shown by in vitro release studies. Intracellular uptake and distribution was studied in HeLa cells using transmission electron microscopy and confocal laser scanning microscopy. This demonstrated liposome internalization and localization in endosomal-lysosomal vesicles. Fluorescence intensity increased in cells exposed to UV light, indicating that this stimulated intracellular drug release; this finding was confirmed by quantitative analyses using flow cytometry. Antitumor efficacy was evaluated in HeLa cells, both in culture and in implants in vivo in nude mice. HeLa cell viability assays showed that light exposure enhanced liposome cytotoxicity and induction of apoptosis. Furthermore, treatment with the prepared liposomes coupled with UV light exposure produced greater antitumor effects in nude mice and reduced side effects, as compared with free Vincristine sulfate.
The effect of antimicrotubule agents on signal transduction pathways of apoptosis: a review.[Pubmed:10501907]
Cancer Chemother Pharmacol. 1999;44(5):355-61.
PURPOSE: Microtubules are important cytoskeletal components involved in many cellular events. Antimicrotubule agents including polymerizing agents (paclitaxel and docetaxel) and depolymerizing drugs (vincristine, vinorelbine, and estramustine phosphate) are widely used either alone or in combination with other anticancer drugs. These antimicrotubule agents are promoters of apoptosis in cancer cells. In this review, we discuss the role of bcl-2 family genes in the regulation of apoptosis, and summarize effects of microtubule targeting agents on apoptotic signal transduction pathways. CONCLUSION: Disruption of microtubule structure by antimicrotubule drugs results in induction of tumor suppressor gene p53 and inhibitor of cyclin-dependent kinases, p21WAF1/CIP1 (p21), and activation/inactivation of several protein kinases including Ras/Raf, PKC/PKA I/II, MAP kinases, and p34cdc2. These protein kinases are associated directly or indirectly with phosphorylation of bcl-2. Phosphorylation of bcl-2 and the elevations of p53 and p21 lead to apoptosis. New pathways of antitumor agents could be directed at this p53, p21 and bcl-2/bax function, and may enhance the effect of existing agents.
Apoptosis induced by microtubule disrupting drugs in cultured human lymphoma cells. Inhibitory effects of phorbol ester and zinc sulphate.[Pubmed:8321748]
Pathol Res Pract. 1993 Mar;189(2):197-203.
The effects of the microtubule disrupting drugs (MDD) vinblastine, vincristine and colchicine on a human lymphoma cell line, BM 13674, were investigated. Twelve hours after administration of vinblastine (10(-3) mg/ml), vincristine (10(-2) mg/ml) or colchicine (10(-2) mg/ml), cell death with the characteristic morphology of apoptosis was observed in 71.6%, 82.2% and 76.9% of the cells respectively. The mode of death was confirmed as apoptotic by the occurrence of internucleosomal DNA cleavage, which was demonstrated by agarose gel electrophoresis. For the purpose of casting light on the mechanism involved, inhibition tests were performed on apoptosis induced by one of these drugs, vinblastine, using a phorbol ester (PDBu), zinc sulphate and cycloheximide. PDBu, an activator of protein kinase C, and zinc sulphate, a putative inhibitor of the endonuclease were thought to be responsible for internucleosomal DNA cleavage; both markedly reduced the induction of apoptosis. The protein synthesis inhibitor cycloheximide, on the other hand, had no inhibitory effect. Moreover, cycloheximide treatment per se enhanced apoptosis. This suggests that new protein synthesis is not required for the execution of vinblastine-induced apoptosis. Such a finding is in accord with recent reports suggesting that the "death program" within many cell types may be primed but unable to proceed due to concomitant production of specific "apoptotic inhibitors". It is suggested that phorbol esters prevent vinblastine-induced apoptosis in the BM 13674 cells by activating one or more of these specific "apoptotic inhibitors", possibly by means of PKC-mediated phosphorylation.
Comparison of the effects of vinblastine, vincristine, vindesine, and vinepidine on microtubule dynamics and cell proliferation in vitro.[Pubmed:3986806]
Cancer Res. 1985 Jun;45(6):2741-7.
Vinepidine, a new derivative of vincristine, and three clinically used Catharanthus derivatives, vinblastine, vincristine, and vindesine, were examined for their abilities to inhibit net tubulin addition at the assembly ends of bovine brain microtubules at steady state. Although all four derivatives were generally similar in potency, their relative abilities to inhibit tubulin addition were distinguishable. Vinepidine and vincristine were the most potent derivatives (Ki, 0.079 +/- 0.018 (SD) microM and 0.085 +/- 0.013 microM, respectively), followed by vindesine (Ki, 0.110 +/- 0.007 microM) and vinblastine (Ki, 0.178 +/- 0.025 microM). In contrast to their relative abilities to inhibit microtubule assembly in vitro, vinblastine and its derivative, vindesine, were generally more potent than vincristine and vinepidine in inhibiting cell proliferation in culture. Vinblastine was nine times more potent than the weakest derivative, vinepidine, in B16 melanoma cells. In L-cells, vinblastine completely inhibited growth at 40 nM, whereas vincristine and vindesine caused about 25% inhibition, and vinepidine was inactive. When B16 melanoma cells were treated with drug before being injected into mice, retardation of tumor growth was best achieved with vindesine, one of the weaker of the four derivatives in vitro. The results demonstrate that chemical differences among the Catharanthus derivatives, which affect to small extents the abilities of the derivatives to inhibit microtubule assembly in vitro, result in significant differences in the order and the magnitude of the abilities of the drugs to inhibit cell growth.


