TriphasiolCAS# 81445-98-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 81445-98-9 | SDF | Download SDF |
| PubChem ID | 157953 | Appearance | Oil |
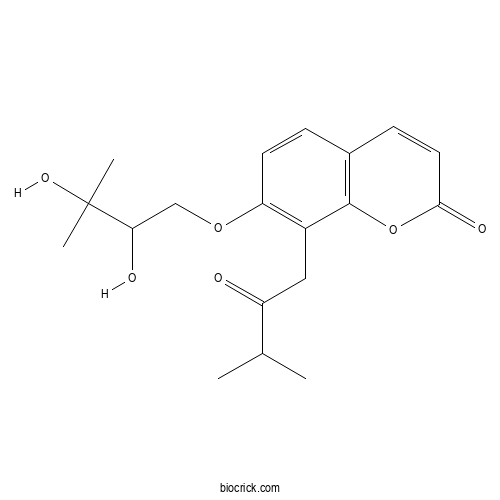
| Formula | C19H24O6 | M.Wt | 348.4 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 7-(2,3-dihydroxy-3-methylbutoxy)-8-(3-methyl-2-oxobutyl)chromen-2-one | ||
| SMILES | CC(C)C(=O)CC1=C(C=CC2=C1OC(=O)C=C2)OCC(C(C)(C)O)O | ||
| Standard InChIKey | DMSHDRKZHASQRO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H24O6/c1-11(2)14(20)9-13-15(24-10-16(21)19(3,4)23)7-5-12-6-8-17(22)25-18(12)13/h5-8,11,16,21,23H,9-10H2,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Triphasiol Dilution Calculator

Triphasiol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8703 mL | 14.3513 mL | 28.7026 mL | 57.4053 mL | 71.7566 mL |
| 5 mM | 0.5741 mL | 2.8703 mL | 5.7405 mL | 11.4811 mL | 14.3513 mL |
| 10 mM | 0.287 mL | 1.4351 mL | 2.8703 mL | 5.7405 mL | 7.1757 mL |
| 50 mM | 0.0574 mL | 0.287 mL | 0.5741 mL | 1.1481 mL | 1.4351 mL |
| 100 mM | 0.0287 mL | 0.1435 mL | 0.287 mL | 0.5741 mL | 0.7176 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Penthoroside B
Catalog No.:BCX0362
CAS No.:2803716-49-4
- Penthoroside A
Catalog No.:BCX0361
CAS No.:2803716-48-3
- Viridiflorol
Catalog No.:BCX0360
CAS No.:552-02-3
- Wilfordeuphone
Catalog No.:BCX0359
CAS No.:2721399-33-1
- ent-1β,4α-Dihydroxyeudesm-7(11)-en-8-one
Catalog No.:BCX0358
CAS No.:142717-58-6
- Hosenkoside N
Catalog No.:BCX0357
CAS No.:156765-13-8
- (E)-2-Ethylidene-3-methylsuccinimide
Catalog No.:BCX0356
CAS No.:28098-82-0
- Excavatin G
Catalog No.:BCX0355
CAS No.:250293-25-5
- Tripchlorolide
Catalog No.:BCX0354
CAS No.:132368-08-2
- (+)-Epinortrachelogenin
Catalog No.:BCX0353
CAS No.:124988-62-1
- Souliene A
Catalog No.:BCX0352
CAS No.:1338332-32-3
- Isomacrophylloside
Catalog No.:BCX0351
CAS No.:209523-03-5
- Isopropylidenekirenol
Catalog No.:BCX0364
CAS No.:89354-33-6
- Botrytone
Catalog No.:BCX0365
CAS No.:1322069-08-8
- Neriifolin
Catalog No.:BCX0366
CAS No.:466-07-9
- Deacetyltanghinin
Catalog No.:BCX0367
CAS No.:4589-95-1
- 2-Oxocleroda-3,13-dien-15,16-olide
Catalog No.:BCX0368
CAS No.:80454-12-2
- 20(R)-Hydroxypregn-4-en-3-one 20-O-glucoside
Catalog No.:BCX0369
CAS No.:50728-28-4
- 9,10-Dihydroxymegastigma-4,7-dien-3-one
Catalog No.:BCX0370
CAS No.:349642-88-2
- 8-Demethoxyschinilenol
Catalog No.:BCX0371
CAS No.:144398-46-9
- Triptriolide
Catalog No.:BCX0372
CAS No.:137131-18-1
- Salcolin B
Catalog No.:BCX0373
CAS No.:369390-52-3
- 3β,5β,6α-Trihydroxy-7-megastigmen-9-one 3-O-glucoside
Catalog No.:BCX0374
CAS No.:1380443-06-0
- Didemethoxycyclocurcumin
Catalog No.:BCX0375
CAS No.:1042441-12-2
Coumarins, furocoumarins and limonoids of Citrus trifoliata and their effects on human colon adenocarcinoma cell lines.[Pubmed:36097483]
Heliyon. 2022 Aug 29;8(9):e10453.
Citrus trifoliata L. (Chinese or Japanese bitter orange) is a medicinal plant with furocoumarins and limonoids as characteristic secondary metabolites. The bitter taste of the fruit limits its use as food, however, it is applied in Asian traditional medicine for its antiphlogistic effect, to treat digestive ulcers and different gastrointestinal disorders and cancer. The phytochemical composition and pharmacological characteristics of this species have not been fully discovered, nevertheless its potential antiproliferative or cytotoxic effects might be related to furocoumarins or limonoids. Our aim was to isolate and identify secondary metabolites from C. trifoliata peel and seeds and to investigate their bioactivities that might be related to the supposed anticancer effect of the plant. By using different chromatographic methods, six pure compounds (phellopterin (2), scoparone (3), myrsellin (4), Triphasiol (6), umbelliferone (7) and citropten (5,7-dimethoxycoumarin (8)) were isolated from the peel and four (imperatorin (1), auraptene (5), limonin (9) and deacetyl nomilin (10)) from the seeds of C. trifoliata fruits. These compounds are furocoumarin (1, 2), coumarin (3-8), and limonoid derivatives (9, 10). Scoparone (3) has been detected in this species for the first time. The furocoumarins (1-2) showed moderate activity on the human colorectal adenocarcinona tumor cell line COLO 320 in antiproliferative assays and 2 also had remarkable P-glycoprotein inhibitory activity and synergistic effect with doxorubicin. The coumarin 5 showed significant activity on the COLO 320 cell line in antiproliferative assays and P-glycoprotein inhibitory activity in the FACS (fluorescence activated cell sorting) assay.
Sortase A-Inhibitory Coumarins from the Folk Medicinal Plant Poncirus trifoliata.[Pubmed:32996318]
J Nat Prod. 2020 Oct 23;83(10):3004-3011.
Thirteen coumarins (1-13), including five new compounds (1-5), were isolated from the folk medicinal plant Poncirus trifoliata. Combined spectroscopic analyses revealed that coumarins 1-4 are bis-isoprenylated coumarins with diverse oxidation patterns, while 5 is an enantiomeric di-isoprenylated coumarin. The absolute configurations of the stereogenic centers in the isoprenyl chains were assigned through MTPA and MPA methods, and those of the known compounds Triphasiol (6) and ponciol (7) were also assigned using similar methods. These coumarins inhibited significantly Staphylococcus aureus-derived sortase A (SrtA), a transpeptidase responsible for anchoring surface proteins to the peptidoglycan cell wall in Gram-positive bacteria. The present results obtained indicated that the bioactivity and underlying mechanism of action of these coumarins are associated with the inhibition of SrtA-mediated S. aureus adhesion to eukaryotic cell matrix proteins including fibrinogen and fibronectin, thus potentially serving as SrtA inhibitors.


