Versicolactone BCAS# 108885-62-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 108885-62-7 | SDF | Download SDF |
| PubChem ID | 165340645 | Appearance | Powder |
| Formula | C15H20O3 | M.Wt | 248.32 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
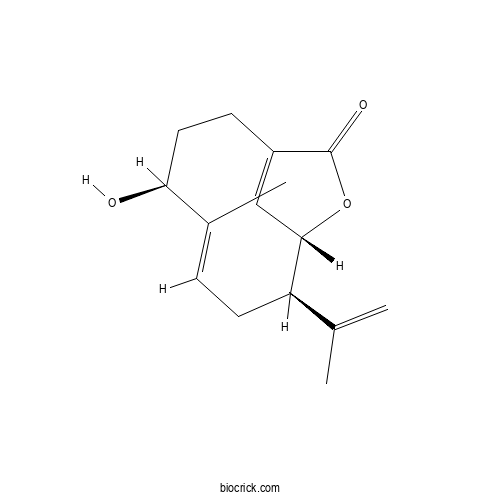
| Chemical Name | (4S,5E,8R,9S)-4-hydroxy-5-methyl-8-prop-1-en-2-yl-10-oxabicyclo[7.2.1]dodeca-1(12),5-dien-11-one | ||
| SMILES | CC1=CCC(C2C=C(CCC1O)C(=O)O2)C(=C)C | ||
| Standard InChIKey | XJUHTEFWFCFCBI-WENQCULDSA-N | ||
| Standard InChI | InChI=1S/C15H20O3/c1-9(2)12-6-4-10(3)13(16)7-5-11-8-14(12)18-15(11)17/h4,8,12-14,16H,1,5-7H2,2-3H3/b10-4+/t12-,13+,14+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Versicolactone B Dilution Calculator

Versicolactone B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0271 mL | 20.1353 mL | 40.2706 mL | 80.5412 mL | 100.6765 mL |
| 5 mM | 0.8054 mL | 4.0271 mL | 8.0541 mL | 16.1082 mL | 20.1353 mL |
| 10 mM | 0.4027 mL | 2.0135 mL | 4.0271 mL | 8.0541 mL | 10.0677 mL |
| 50 mM | 0.0805 mL | 0.4027 mL | 0.8054 mL | 1.6108 mL | 2.0135 mL |
| 100 mM | 0.0403 mL | 0.2014 mL | 0.4027 mL | 0.8054 mL | 1.0068 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Madolin U
Catalog No.:BCX0378
CAS No.:327185-00-2
- Calquiquelignan E
Catalog No.:BCX0377
CAS No.:1292294-31-5
- Calquiquelignan D
Catalog No.:BCX0376
CAS No.:1928715-38-1
- Didemethoxycyclocurcumin
Catalog No.:BCX0375
CAS No.:1042441-12-2
- 3β,5β,6α-Trihydroxy-7-megastigmen-9-one 3-O-glucoside
Catalog No.:BCX0374
CAS No.:1380443-06-0
- Salcolin B
Catalog No.:BCX0373
CAS No.:369390-52-3
- Triptriolide
Catalog No.:BCX0372
CAS No.:137131-18-1
- 8-Demethoxyschinilenol
Catalog No.:BCX0371
CAS No.:144398-46-9
- 9,10-Dihydroxymegastigma-4,7-dien-3-one
Catalog No.:BCX0370
CAS No.:349642-88-2
- 20(R)-Hydroxypregn-4-en-3-one 20-O-glucoside
Catalog No.:BCX0369
CAS No.:50728-28-4
- 2-Oxocleroda-3,13-dien-15,16-olide
Catalog No.:BCX0368
CAS No.:80454-12-2
- Deacetyltanghinin
Catalog No.:BCX0367
CAS No.:4589-95-1
- Diferuloylputrescine
Catalog No.:BCX0380
CAS No.:42369-86-8
- Oxytroflavoside G
Catalog No.:BCX0381
CAS No.:1391144-89-0
- Deacetylnomilin
Catalog No.:BCX0382
CAS No.:3264-90-2
- Triptotriterpenic acid C
Catalog No.:BCX0383
CAS No.:123914-32-9
- Asperazine
Catalog No.:BCX0384
CAS No.:198953-76-3
- Gnetuhainin I
Catalog No.:BCX0385
CAS No.:308105-06-8
- Malformin C
Catalog No.:BCX0386
CAS No.:59926-78-2
- Sclerone
Catalog No.:BCX0387
CAS No.:19638-58-5
- 14,15,16-Trinorlabda-8(17),11-dien-13-oic acid
Catalog No.:BCX0388
CAS No.:917078-12-7
- 4,4'-(1,3-Dimethylbutylidene)diphenol
Catalog No.:BCX0389
CAS No.:6807-17-6
- (2E,6E)-Farnesyl acetate
Catalog No.:BCX0390
CAS No.:4128-17-0
- Excoecafolin C
Catalog No.:BCX0391
CAS No.:1643370-00-6
Butenolides from a marine-derived fungus Aspergillus terreus with antitumor activities against pancreatic ductal adenocarcinoma cells.[Pubmed:30392953]
Bioorg Med Chem. 2018 Dec 1;26(22):5903-5910.
Chemical study on the extract of a marine-derived fungus Aspergillus terreus yielded twelve butenolide derivatives, including three new compounds, namely asperlides A-C (1-3) and nine known butenolides (4-12). The structures of 1-3 were confirmed by comprehensive spectroscopic analysis, including HRESIMS, NMR spectroscopy, and calculated electronic circular dichroism (ECD). The cytotoxicity of the compounds was evaluated using PANC-1, HCC1806, HepG2, BEAS-2B and HT-29 cancer cells. The results showed that (+)-3',3'-di-(dimethylallyl)-butyrolactone II (4) and Versicolactone B (6) exhibited the most potent cytotoxin of PANC-1 cell line, with the IC(50) values of 5.3 and 9.4 muM, respectively. Morphological features of apoptosis were observed in 4 and 6-treated PANC-1 cells, including apoptotic body formation, membrane blebbing, cell shrinkage and nuclear condensation. Cell cycle analysis with propidium iodide staining exhibited that 4 inhibits proliferation of PANC-1 cells via the induction of G(2)/M and S phase arrest, while 6 could retard the PANC-1 cells via the induction of S phase arrest. Flow cytometric analysis suggested that treatment with 4 and 6 significantly induced PANC-1 cells apoptosis. These findings indicated that 4 and 6 might serve as a starting point for the development of an anticancer drug for the treatment of pancreatic ductal adenocarcinoma.
Anti-complement sesquiterpenes from Viola yedoensis.[Pubmed:25562805]
Fitoterapia. 2015 Mar;101:73-9.
Two new germacrane sesquiterpenes, yedoensins A (1) and B (2), together with 8 known ones (3-10) were isolated from the herb of Viola yedoensis. The structures of the new compounds were established by extensive spectroscopic means including 1D ((1)H and (13)C) and 2D NMR experiments (HSQC, HMBC, and NOESY) as well as HR-ESI-MS analysis. The absolute configurations of the known sesquiterpenes Versicolactone B (3) and madolin W (6) were determined by a modified Mosher's method for the first time. The sesquiterpenes 1-3, and 5-9 exhibited anti-complement activity against the classical pathway (CP) and the alternative pathway (AP) with the CH50 and AP50 values ranging from 0.14 to 0.37mg/mL and 0.32 to 0.54mg/mL, respectively. Preliminary mechanism study using complement-depleted sera showed that yedoensin A (1) and Versicolactone B (3) acted on C1q, C3 and C9, while madolin W (6), aristoyunnolin E (7) and madolin Y (9) interacted with C1q, C3, C5 and C9 components in the complement activation cascade.


