Xanthoangelol FCAS# 265652-71-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 265652-71-9 | SDF | Download SDF |
| PubChem ID | 6479088 | Appearance | Powder |
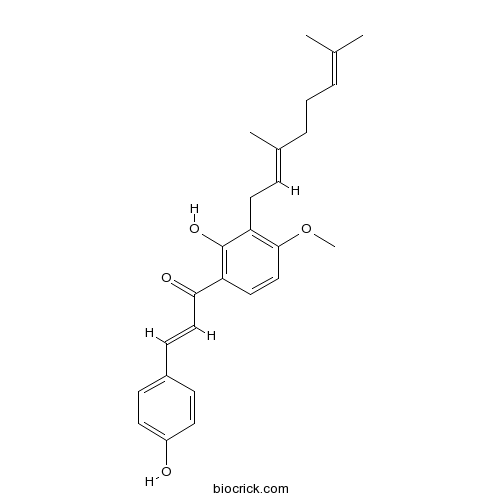
| Formula | C26H30O4 | M.Wt | 406.52 |
| Type of Compound | Chalcones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (E)-1-[3-[(2E)-3,7-dimethylocta-2,6-dienyl]-2-hydroxy-4-methoxyphenyl]-3-(4-hydroxyphenyl)prop-2-en-1-one | ||
| SMILES | CC(=CCCC(=CCC1=C(C=CC(=C1O)C(=O)C=CC2=CC=C(C=C2)O)OC)C)C | ||
| Standard InChIKey | XBFSDEKOTLYPJU-SXZUIPJJSA-N | ||
| Standard InChI | InChI=1S/C26H30O4/c1-18(2)6-5-7-19(3)8-14-23-25(30-4)17-15-22(26(23)29)24(28)16-11-20-9-12-21(27)13-10-20/h6,8-13,15-17,27,29H,5,7,14H2,1-4H3/b16-11+,19-8+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Xanthoangelol F showed strong PTP1B inhibitory effect with the IC50 values of 1.67 μg/mL. It inhibited phenylephrine-induced vasoconstriction through endothelium-dependent production of EDRF/NO and/or through the reduction of the [Ca2+]i elevation induced by phenylephrine. |
| Targets | PTP1B | Calcium Channel | NO | EDRF |
| In vitro | Chalcones from Angelica keiskei: Evaluation of Their Heat Shock Protein Inducing Activities.[Pubmed: 26431394]J Nat Prod. 2015 Oct 23;78(10):2481-7
Artery relaxation by chalcones isolated from the roots of Angelica keiskei.[Pubmed: 11345693 ]Planta Med. 2001 Apr;67(3):230-5.An EtOAc-soluble fraction from a 50% EtOH extract of the roots of Angelica keiskei inhibited phenylephrine-induced vasoconstriction in rat aortic rings, while an EtOAc-insoluble fraction had no effect at 100 micrograms/ml. |
| Kinase Assay | PTP1B inhibitors from stems of Angelica keiskei (Ashitaba).[Pubmed: 25891102 ]Bioorg Med Chem Lett. 2015;25(10):2028-32.Three new chalcones, xanthoangelols K-M (1-3), together with 19 known compounds were isolated from the stems of Angelica keiskei Koidzumi, a well-known rejuvenated and anti-diabetic plant originated from Japan.
|

Xanthoangelol F Dilution Calculator

Xanthoangelol F Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4599 mL | 12.2995 mL | 24.599 mL | 49.1981 mL | 61.4976 mL |
| 5 mM | 0.492 mL | 2.4599 mL | 4.9198 mL | 9.8396 mL | 12.2995 mL |
| 10 mM | 0.246 mL | 1.23 mL | 2.4599 mL | 4.9198 mL | 6.1498 mL |
| 50 mM | 0.0492 mL | 0.246 mL | 0.492 mL | 0.984 mL | 1.23 mL |
| 100 mM | 0.0246 mL | 0.123 mL | 0.246 mL | 0.492 mL | 0.615 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Betulinic acid methyl ester
Catalog No.:BCN6378
CAS No.:2259-06-5
- Lucidenic acid L
Catalog No.:BCN6389
CAS No.:110267-45-3
- Neochebulagic acid
Catalog No.:BCN7368
CAS No.:28196-46-5
- Chelidimerine
Catalog No.:BCN8410
CAS No.:39110-99-1
- Tuberosin
Catalog No.:BCN8735
CAS No.:41347-45-9
- Picropodopyllotoxone
Catalog No.:BCN7574
CAS No.:477-48-5
- 6''-O-Acetylsaikosaponin D
Catalog No.:BCN6392
CAS No.:64340-45-0
- Polygalasaponin XLIX
Catalog No.:BCN8470
CAS No.:1033593-12-2
- Physalin G
Catalog No.:BCN7815
CAS No.:76045-38-0
- Guvacine
Catalog No.:BCN6529
CAS No.:498-96-4
- Luteolin-4'-O-glucoside
Catalog No.:BCN8734
CAS No.:6920-38-3
- 1,3,6-Trihydroxy-2-methylanthraquinone 3-O-(6'-O-acetyl)-alpha-L-rhamnosyl-(1->2)-Beta-D-glucoside
Catalog No.:BCN6384
CAS No.:87686-87-1
- 6''-O-acetylsaikosaponin A
Catalog No.:BCN6478
CAS No.:64340-46-1
- Kaempferol 3-O-beta-(6''-p-coumaroyl)glucopyranosyl(1->2)-alpha-L-rhamnopyranoside
Catalog No.:BCN7711
CAS No.:111957-48-3
- Orcinol 1-O-beta-D-apiofuranosyl-(1->6)-beta-D-glucopyranoside
Catalog No.:BCN8273
CAS No.:868557-54-4
- Luteolin 7-rutinoside
Catalog No.:BCN6360
CAS No.:20633-84-5
- 3'-Angeloyloxy-4'-senecioyloxy-2',3'-dihydrooroselol
Catalog No.:BCN8730
CAS No.:1221686-60-7
- Cyclo(Hyp-Val)
Catalog No.:BCN6391
CAS No.:1425501-89-8
- 3'-Methoxymirificin
Catalog No.:BCN6377
CAS No.:1297609-29-0
- 2-Cinnamoyl-1-galloylglucose
Catalog No.:BCN6383
CAS No.:56994-83-3
- Quinizarin monoglucoside
Catalog No.:BCN6382
CAS No.:39115-11-2
- Cassiaside C
Catalog No.:BCN8733
CAS No.:119170-52-4
- Armepavine
Catalog No.:BCN8490
CAS No.:524-20-9
- Suavissimoside F1
Catalog No.:BCN6361
CAS No.:95645-51-5
Chalcones from Angelica keiskei: Evaluation of Their Heat Shock Protein Inducing Activities.[Pubmed:26431394]
J Nat Prod. 2015 Oct 23;78(10):2481-7.
Five new chalcones, 4,2',4'-trihydroxy-3'-[(2E,5E)-7-methoxy-3,7-dimethyl-2,5-octadienyl]chalcone (1), (+/-)-4,2',4'-trihydroxy-3'-[(2E)-6-hydroxy-7-methoxy-3,7-dimethyl-2-octenyl]chal cone (2), 4,2',4'-trihydroxy-3'-[(2E)-3-methyl-5-(1,3-dioxolan-2-yl)-2-pentenyl]chalcone (3), 2',3'-furano-4-hydroxy-4'-methoxychalcone (4), and (+/-)-4-hydroxy-2',3'-(2,3-dihydro-2-methoxyfurano)-4'-methoxychalcone (5), were isolated from the aerial parts of Angelica keiskei Koidzumi together with eight known chalcones, 6-13, which were identified as (+/-)-4,2',4'-trihydroxy-3'-[(6E)-2-hydroxy-7-methyl-3-methylene-6-octenyl]chalco ne (6), xanthoangelol (7), Xanthoangelol F (8), xanthoangelol G (9), 4-hydroxyderricin (10), xanthoangelol D (11), xanthoangelol E (12), and xanthoangelol H (13), respectively. Chalcones 1-13 were evaluated for their promoter activity on heat shock protein 25 (hsp25, murine form of human hsp27). Compounds 1 and 6 activated the hsp25 promoter by 21.9- and 29.2-fold of untreated control at 10 muM, respectively. Further protein expression patterns of heat shock factor 1 (HSF1), HSP70, and HSP27 by 1 and 6 were examined. Compound 6 increased the expression of HSF1, HSP70, and HSP27 by 4.3-, 1.5-, and 4.6-fold of untreated control, respectively, without any significant cellular cytotoxicities, whereas 1 did not induce any expression of these proteins. As a result, 6 seems to be a prospective HSP inducer.
PTP1B inhibitors from stems of Angelica keiskei (Ashitaba).[Pubmed:25891102]
Bioorg Med Chem Lett. 2015;25(10):2028-32.
Three new chalcones, xanthoangelols K-M (1-3), together with 19 known compounds were isolated from the stems of Angelica keiskei Koidzumi, a well-known rejuvenated and anti-diabetic plant originated from Japan. The structures of compounds 1-3 were elucidated on the basis of spectroscopic data and Mosher's method. All compounds were evaluated for their inhibitory activity against protein tyrosine phosphatase 1B (PTP1B). Among them, six chalcones, xanthoangelol K (1), xanthoangelol (4), Xanthoangelol F (5), 4-hydroxyderricin (6), xanthoangelol D (7), xanthoangelol E (8), and a coumarin, methoxsalen (17), showed strong PTP1B inhibitory effect with IC50 values of 0.82, 1.97, 1.67, 2.47, 3.97, 1.43, and 2.53mug/mL, respectively. A kinetic study revealed that compound 1 inhibited PTP1B with characteristics typical of a competitive inhibitor. Molecular docking simulations elucidated that ring B of 1 may anchor in a pocket of PTP1B and the molecule is stabilized by hydrogen bonds with Arg47, Asp48, and pi-pi interaction with Phe182 of PTP1B.
Cytotoxic activities and anti-tumor-promoting effects of microbial transformation products of prenylated chalcones from Angelica keiskei.[Pubmed:22344908]
Chem Biodivers. 2012 Feb;9(2):318-30.
Three prenylated chalcones, 4-hydroxyderricin (1), xanthoangelol (2), and Xanthoangelol F (3), isolated from Angelica keiskei, were transformed by the fungus Aspergillus saitoi. These chalcones were converted to flavanones (i.e., 4, 8, and 12), and prenyl-chain-hydrated (i.e., 5, 7, 9-11, and 13) and ring-B-hydroxylated (i.e., 6) chalcones. The structures of three new metabolites, 7, 9, and 13, were established as 2'',3''-dihydro-4,3''-dihydroxyderricin, 6'',7''-dihydro-7''-hydroxyxanthoangelol, and 6'',7''-dihydro-7''-hydroxyXanthoangelol F, respectively. Upon evaluation of cytotoxic activities of compounds 1-13, the metabolite 7 exhibited potent cytotoxicity against HL60 cells, and this cell death was revealed to be mostly due to apoptosis. In addition, compounds 1-4, 7-10, 12, and 13 were examined for their inhibitory effects on the induction of Epstein-Barr virus early antigen (EBV-EA) by 12-O-tetradecanoylphorbol-13-acetate (TPA) in Raji cells. All compounds tested showed inhibitory effects against EBV-EA activation with potencies higher than that of beta-carotene. Furthermore, the metabolite 13 exhibited inhibitory effect on skin tumor promotion in an in vivo two-stage mouse skin carcinogenesis test based on 7,12-dimethylbenz[a]anthracene (DMBA) as initiator, and with TPA as promoter.


