β-PompilidotoxinSlows Na+ channel inactivation CAS# 216064-36-7 |
- Laminin (925-933)
Catalog No.:BCC1015
CAS No.:110590-60-8
- Epidermal Growth Factor Receptor Peptide (985-996)
Catalog No.:BCC1014
CAS No.:96249-43-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 216064-36-7 | SDF | Download SDF |
| PubChem ID | 90479801 | Appearance | Powder |
| Formula | C71H124N22O17 | M.Wt | 1557.9 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | RIKIGLFDQLSRL-NH2; Beta-Pompilidotoxin; b-Pompilidotoxin | ||
| Solubility | Soluble to 1 mg/ml in water | ||
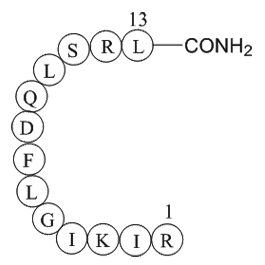
| Sequence | RIKIGLFDQLSRL (Modifications: Leu-13 = C-terminal amide) | ||
| Chemical Name | (3S)-3-[[(2S)-2-[[(2S)-2-[[2-[[(2S,3S)-2-[[(2S)-6-amino-2-[[(2S,3S)-2-[[(2S)-2-amino-5-(diaminomethylideneamino)pentanoyl]amino]-3-methylpentanoyl]amino]hexanoyl]amino]-3-methylpentanoyl]amino]acetyl]amino]-4-methylpentanoyl]amino]-3-phenylpropanoyl]amino]-4-[[(2S)-5-amino-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-amino-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-hydroxy-1-oxopropan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-1,5-dioxopentan-2-yl]amino]-4-oxobutanoic acid | ||
| SMILES | CCC(C)C(C(=O)NC(CCCCN)C(=O)NC(C(C)CC)C(=O)NCC(=O)NC(CC(C)C)C(=O)NC(CC1=CC=CC=C1)C(=O)NC(CC(=O)O)C(=O)NC(CCC(=O)N)C(=O)NC(CC(C)C)C(=O)NC(CO)C(=O)NC(CCCN=C(N)N)C(=O)NC(CC(C)C)C(=O)N)NC(=O)C(CCCN=C(N)N)N | ||
| Standard InChIKey | YBOJYGJMKPMNRC-QRIWDNSUSA-N | ||
| Standard InChI | InChI=1S/C71H124N22O17/c1-11-40(9)56(93-62(103)44(23-16-17-27-72)86-69(110)57(41(10)12-2)92-59(100)43(73)22-18-28-80-70(76)77)68(109)82-35-54(96)83-48(31-38(5)6)63(104)89-50(33-42-20-14-13-15-21-42)65(106)90-51(34-55(97)98)66(107)85-46(25-26-53(74)95)61(102)88-49(32-39(7)8)64(105)91-52(36-94)67(108)84-45(24-19-29-81-71(78)79)60(101)87-47(58(75)99)30-37(3)4/h13-15,20-21,37-41,43-52,56-57,94H,11-12,16-19,22-36,72-73H2,1-10H3,(H2,74,95)(H2,75,99)(H,82,109)(H,83,96)(H,84,108)(H,85,107)(H,86,110)(H,87,101)(H,88,102)(H,89,104)(H,90,106)(H,91,105)(H,92,100)(H,93,103)(H,97,98)(H4,76,77,80)(H4,78,79,81)/t40-,41-,43-,44-,45-,46-,47-,48-,49-,50-,51-,52-,56-,57-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Novel wasp neurotoxin that slows Na+ channel inactivation. Facilitates neuromuscular synaptic transmission and discriminates between rat neuronal and cardiac Na+ channel α-subunits. |

β-Pompilidotoxin Dilution Calculator

β-Pompilidotoxin Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
β-Pompilidotoxin, (C71H124N22O17), a peptide with the sequence H2N-Arg
-Ile-Lys-Ile-Gly-Leu-Phe-Asp-Gln-Leu-Ser-Arg-Leu-amide, MW= 1557.88. Pompilidotoxin is a toxin from the venom of spider wasps that slows the inactivation of Na+channels. α-Pompilidotoxin (α-PMTX) can be extracted from the venom of a solitary wasp, Anopolis samariensis. β-Pompilidotoxin (β-PMTX) originates from the venom of another wasp, Batozonellus maculifrons(1). Homology α-PMTX has no structural homology with other toxins. It lacks disulfide bonds which are known to be present in other toxins acting on sodium channels, such as sea anemone toxins or scorpion toxins(2). Both α- and β-PMTX slow the inactivation of neuronal sodium channels (but not heart sodium channels), possibly by binding to the neurotoxin receptor site 3 on the extracellular surface of the sodium channel(3). β-PMTX has higher potency than α-PMTX. By slowing down the inactivation of sodium channels, PMTXs can potentiate synaptic transmission in the lobster neuromuscular endplate.
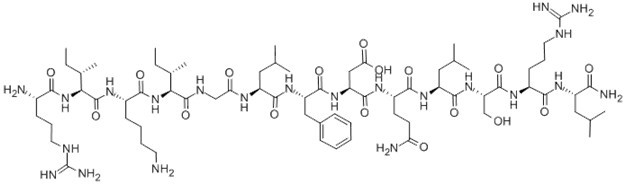
Figure1 Formula of β-Pompilidotoxin
Ref:
1. Konno K, Hisada M, Itagaki Y, Naoki H, Kawai N, Miwa A, Yasuhara T, Takayama H (1998). "Isolation and structure of pompilidotoxins, novel peptide neurotoxins in solitary wasp venoms". Biochem Biophys Res Commun. 250 (3): 612–6.
2. Sahara Y, Gotoh M, Konno K, Miwa A, Tsubokawa H, Robinson HP, Kawai N (2000). "A new class of neurotoxin from wasp venom slows inactivation of sodium current". Eur J Neurosci. 12 (6): 1961–70.
3. Kawai N, Konno K (2004). "Molecular determinants of two neurotoxins that regulate sodium current inactivation in rat hippocampal neurons". Neurosci Lett. 361 (1-3): 44–6.
- 1-Methyl-3-nitrophthalate
Catalog No.:BCC8468
CAS No.:21606-04-2
- 15,16-Epoxy-12S-hydroxylabda-8(17),13(16),14-triene
Catalog No.:BCN1491
CAS No.:216011-55-1
- 7-Hydroxy-beta-carboline-1-propionic acid
Catalog No.:BCN1492
CAS No.:215934-15-9
- CX 546
Catalog No.:BCC7532
CAS No.:215923-54-9
- Bruceine E
Catalog No.:BCN7619
CAS No.:21586-90-3
- SB-277011
Catalog No.:BCC1928
CAS No.:215803-78-4
- SB269652
Catalog No.:BCC8052
CAS No.:215802-15-6
- Cyclocephaloside II
Catalog No.:BCC8310
CAS No.:215776-78-6
- 23-deoxojessic acid
Catalog No.:BCN4926
CAS No.:215609-93-1
- Sodium Dichloroacetate
Catalog No.:BCN2951
CAS No.:2156-56-1
- SU 5402
Catalog No.:BCC1970
CAS No.:215543-92-3
- Mianserin HCl
Catalog No.:BCC1114
CAS No.:21535-47-7
- Bis(2,6-diisopropylphenyl)carbodiimide
Catalog No.:BCC8879
CAS No.:2162-74-5
- 1alpha, 25-Dihydroxy VD2-D6
Catalog No.:BCC1299
CAS No.:216244-04-1
- α-Conotoxin AuIB
Catalog No.:BCC5975
CAS No.:216299-21-7
- 3',4',5',3,5,7,8-Heptamethoxyflavone
Catalog No.:BCN4927
CAS No.:21634-52-6
- Isoquerglanin
Catalog No.:BCC8189
CAS No.:143519-53-3
- N-Demethylricinine
Catalog No.:BCC9098
CAS No.:21642-98-8
- Sophoflavescenol
Catalog No.:BCN2891
CAS No.:216450-65-6
- Bisphenol P
Catalog No.:BCC8891
CAS No.:2167-51-3
- Shoreic acid
Catalog No.:BCN4928
CAS No.:21671-00-1
- Fludarabine
Catalog No.:BCC2518
CAS No.:21679-14-1
- Kulinone
Catalog No.:BCN7954
CAS No.:21688-61-9
- Lauroscholtzine
Catalog No.:BCN4929
CAS No.:2169-44-0
Production of resurgent current in NaV1.6-null Purkinje neurons by slowing sodium channel inactivation with beta-pompilidotoxin.[Pubmed:14715935]
J Neurosci. 2004 Jan 7;24(1):35-42.
Voltage-gated tetrodotoxin-sensitive sodium channels of Purkinje neurons produce "resurgent" current with repolarization, which results from relief of an open-channel block that terminates current flow at positive potentials. The associated recovery of sodium channels from inactivation is thought to facilitate the rapid firing patterns characteristic of Purkinje neurons. Resurgent current appears to depend primarily on NaV1.6 alpha subunits, because it is greatly reduced in "med" mutant mice that lack NaV1.6. To identify factors that regulate the susceptibility of alpha subunits to open-channel block, we voltage clamped wild-type and med Purkinje neurons before and after slowing conventional inactivation with beta-pompilidotoxin (beta-PMTX). beta-PMTX increased resurgent current in wild-type neurons and induced resurgent current in med neurons. In med cells, the resurgent component of beta-PMTX-modified sodium currents could be selectively abolished by application of intracellular alkaline phosphatase, suggesting that, like in NaV1.6-expressing cells, the open-channel block of NaV1.1 and NaV1.2 subunits is regulated by constitutive phosphorylation. These results indicate that the endogenous blocker exists independently of NaV1.6 expression, and conventional inactivation regulates resurgent current by controlling the extent of open-channel block. In Purkinje cells, therefore, the relatively slow conventional inactivation kinetics of NaV1.6 appear well adapted to carry resurgent current. Nevertheless, NaV1.6 is not unique in its susceptibility to open-channel block, because under appropriate conditions, the non-NaV1.6 subunits can produce robust resurgent currents.
Modulation of synaptic transmission in hippocampal CA1 neurons by a novel neurotoxin (beta-pompilidotoxin) derived from wasp venom.[Pubmed:11755223]
Neurosci Res. 2001 Dec;41(4):365-71.
We examined the effects of beta-pompilidotoxin (beta-PMTX), a neurotoxin derived from wasp venom, on synaptic transmission in the mammalian central nervous system (CNS). Using hippocampal slice preparations of rodents, we made both extracellular and intracellular recordings from the CA1 pyramidal neurons in response to stimulation of the Schaffer collateral/commissural fibers. Application of 5-10 microM beta-PMTX enhanced excitatory postsynaptic potentials (EPSPs) but suppressed the fast component of the inhibitory postsynaptic potentials (IPSPs). In the presence of 10 microM bicuculline, beta-PMTX potentiated EPSPs that were composed of both non-NMDA and NMDA receptor-mediated potentials. Potentiation of EPSPs was originated by repetitive firings of the presynaptic axons, causing summation of EPSPs. In the presence of 10 microM CNQX and 50 microM APV, beta-PMTX suppressed GABA(A) receptor-mediated fast IPSPs but retained GABA(B) receptor-mediated slow IPSPs. Our results suggest that beta-PMTX facilitates excitatory synaptic transmission by a presynaptic mechanism and that it causes overexcitation followed by block of the activity of some population of interneurons which regulate the activity of GABA(A) receptors.
beta-pompilidotoxin modulates spontaneous activity and persistent sodium currents in spinal networks.[Pubmed:20955768]
Neuroscience. 2011 Jan 13;172:129-38.
The origin of rhythm generation in mammalian spinal cord networks is still poorly understood. In a previous study, we showed that spontaneous activity in spinal networks takes its origin in the properties of certain intrinsically spiking interneurons based on the persistent sodium current (INaP). We also showed that depolarization block caused by a fast inactivation of the transient sodium current (INaT) contributes to the generation of oscillatory activity in spinal cord cultures. Recently, a toxin called beta-pompilidotoxin (beta-PMTX) that slows the inactivation process of tetrodotoxin (TTX)-sensitive sodium channels has been extracted from the solitary wasp venom. In the present study, we therefore investigated the effect of beta-PMTX on rhythm generation and on sodium currents in spinal networks. Using intracellular recordings and multielectrode array (MEA) recordings in dissociated spinal cord cultures from embryonic (E14) rats, we found that beta-PMTX reduces the number of population bursts and increases the background asynchronous activity. We then uncoupled the network by blocking all synaptic transmission (APV, CNQX, bicuculline and strychnine) and observed that beta-PMTX increases both the intrinsic activity at individual channels and the number of intrinsically activated channels. At the cellular level, we found that beta-PMTX has two effects: it switches 58% of the silent interneurons into spontaneously active interneurons and increases the firing rate of intrinsically spiking cells. Finally, we investigated the effect of beta-PMTX on sodium currents. We found that this toxin not only affects the inactivation of INaT but also increases the peak amplitude of the persistent sodium current (INaP). Altogether, theses findings suggest that beta-PMTX acting on INaP and INaT enhances intrinsic activity leading to a profound modulation of spontaneous rhythmic activity in spinal networks.
Differential effects of novel wasp toxin on rat hippocampal interneurons.[Pubmed:12123851]
Neurosci Lett. 2002 Aug 2;328(1):25-8.
We studied the effects of a wasp toxin beta-pompilidotoxin (beta-PMTX) on rat hippocampal CA1 interneurons by the current-clamp technique. The firing patterns of pyramidal neurons and pyramidale interneurons were not affected by beta-PMTX, but in oriens and radiatum interneurons, beta-PMTX converted the action potentials to prolonged depolarizing potentials by slowing the inactivation of Na(+) channels. In lacunosum moleculare interneurons, beta-PMTX induced initial bursting spikes followed by block of succeeding spikes. Comparison of beta-PMTX with a sea anemone toxin, ATX II, revealed that ATX II altered the firing properties of pyramidal neurons and pyramidale interneurons that were unchanged by beta-PMTX. Our results suggest that beta-PMTX modulates Na(+) currents in CA1 interneurons differently in various CA1 neurons and the toxin is useful to classify Na(+) channel subtypes.
Novel wasp toxin discriminates between neuronal and cardiac sodium channels.[Pubmed:11353806]
Mol Pharmacol. 2001 Jun;59(6):1457-63.
Pompilidotoxins (PMTXs), derived from the venom of solitary wasp has been known to facilitate synaptic transmission in the lobster neuromuscular junction, and a recent further study from rat trigeminal neurons revealed that the toxin slows Na+ channel inactivation without modifying activation process. Here we report that beta-PMTX modifies rat brain type II Na+ channel alpha-subunit (rBII) expressed in human embryonic kidney cells but fails to act on the rat heart alpha-subunit (rH1) at similar concentrations. We constructed a series of chimeric mutants of rBII and rH1 Na+ channels and compared modification of the steady-state Na+ currents by beta-PMTX. We found that a difference in a single amino acid between Glu-1616 in rBII and Gln-1615 in rH1 at the extracellular loop of D4S3-S4 is crucial for the action of beta-PMTX. PMTXs, which are small peptides with 13 amino acids, would be a potential tool for exploring a new functional moiety of Na+ channels.
Isolation and structure of pompilidotoxins, novel peptide neurotoxins in solitary wasp venoms.[Pubmed:9784394]
Biochem Biophys Res Commun. 1998 Sep 29;250(3):612-6.
Novel peptide neurotoxins, alpha- and beta-pompilidotoxins (alpha- and beta-PMTXs), were purified from the venoms of the solitary wasps Anoplius samariensis and Batozonellus maculifrons. Their structures were analyzed mostly by MALDI-TOF-MS, which were corroborated by solid-phase synthesis. alpha-PMTX, with 13 amino acid residues and the sequence of Arg-Ile-Lys-Ile-Gly-Leu-Phe-Gln-Asp-Leu-Ser-Lys-Leu-NH2, greatly potentiates synaptic transmission of lobster leg muscle by the presynaptic mechanisms. beta-PMTX, in which the lysine residue at 12 position of alpha-PMTX was replaced with arginine, was more potent than alpha-PMTX.


