Anemarrhena asphodeloides
Anemarrhena asphodeloides
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
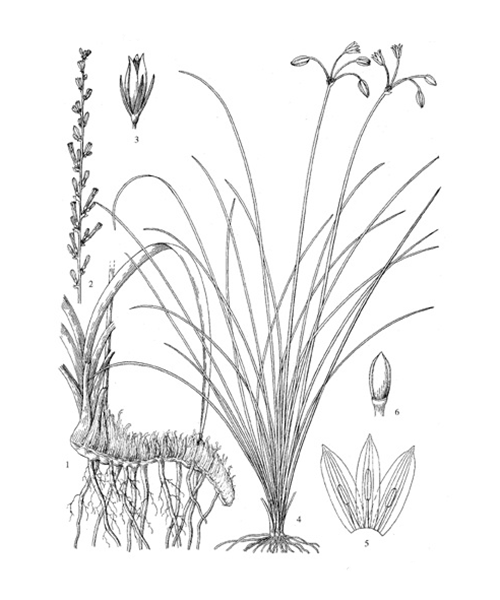
Natural products/compounds from Anemarrhena asphodeloides
- Cat.No. Product Name CAS Number COA
-
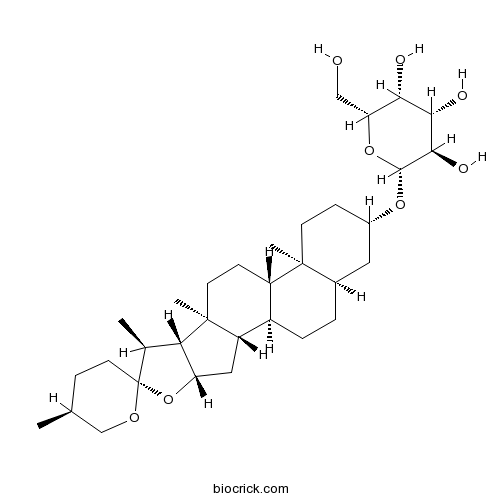
BCN1269
Sarsasapogenin126-19-2
Instructions

-
BCN6290
Anemarsaponin E136565-73-6
Instructions

-
BCN4998
Timosaponin BII136656-07-0
Instructions

-
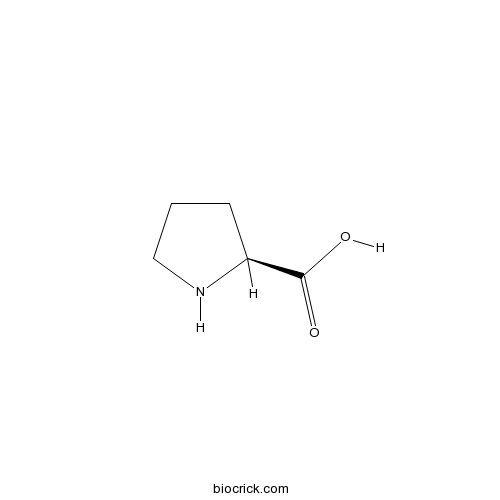
BCN1656
Proline147-85-3
Instructions
-
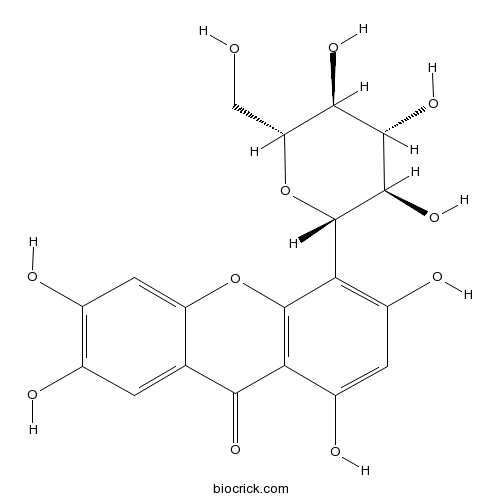
BCN2528
Isomangiferin24699-16-9
Instructions
-
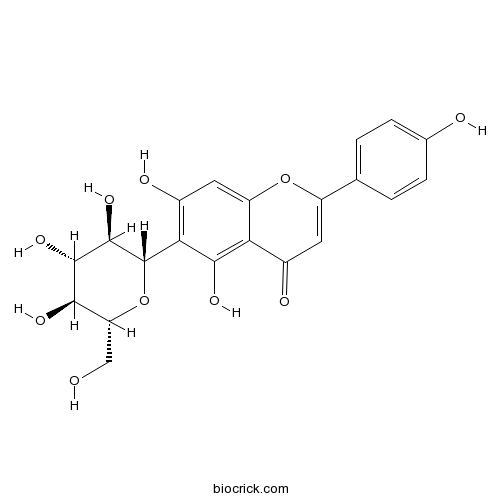
BCN5423
Vitexin3681-93-4
Instructions

-
BCN5441
Isovitexin38953-85-4
Instructions
-
BCN4999
Timosaponin A341059-79-4
Instructions

-
BCN5535
Mangiferin4773-96-0
Instructions

-
BCN4970
Neomangiferin64809-67-2
Instructions

-
BCN7819
Timosaponin AI68422-00-4
Instructions
-
BCC3138
H-Val-OH72-18-4
Instructions

Gas chromatography-mass spectrometry-based trimethylsilyl-alditol derivatives for quantitation and fingerprint analysis of Anemarrhena asphodeloides Bunge polysaccharides.[Pubmed: 30092985]
Here we report a novel approach using gas chromatography mass spectrometry (GCMS)-based trimethylsilyl-alditol (TMSA) derivatives for simultaneous baseline separation and detection of 8 neutral saccharides and 2 uronic acids within 25 min. Using mild alkaline conditions to dissolve the sample in advance significantly increased both the detection sensitivity and sample stability of uronic acids because of occurrence of de-lactonization, whereas no obvious effects were observed for neutral saccharides. Sodium borohydride reduction of the carbonyl group of aldoses and the subsequent formation of TMSA derivatives simplifies GCMS chromatograms by producing a single peak for each derivatized sugar. The effects of both reaction temperatures and solvent ratios between HMDS and TMCS on formations of TMSA derivatives were also investigated. The established GCMS method was successfully applied for quantitation and fingerprint analysis of polysaccharides from the plant Anemarrhena asphodeloides Bunge. A comparative analysis of A. asphodeloides polysaccharides was further performed between TMSA and other four types of derivatizations. The results showed that GCMS analysis based on precolumn TMSA derivatization coupled with fingerprint analysis is a comprehensive and effective technique for qualitative and quantitative analysis of plant polysaccharides from traditional Chinese medicines.
Protective effects of sarsasapogenin against early stage of diabetic nephropathy in rats.[Pubmed: 29682805]
Rhizome of Anemarrhena asphodeloides Bunge (AA, family Liliaceae) has been widely used in China for thousands of years to treat febrile diseases and diabetes. Steroidal saponins from AA show good antidiabetes effects and ameliorate diabetic complications. This study was designed to investigate the effects of sarsasapogenin (Sar), a major sapogenin from AA, on diabetic nephropathy (DN) in rats, and to explore the possible mechanisms. Diabetic rats were divided into 3 groups treated orally with Sar (0, 20, or 60 mg/kg) and carboxymethylcellulose sodium, whereas normal rats for Sar (0 or 60 mg/kg) and carboxymethylcellulose sodium. We found that chronic treatment with Sar for 9 weeks significantly ameliorated renal dysfunction of diabetic rats, as evidenced by decreases in albuminuria, kidney weight index, serum uric acid, and morphologic changes such as extracellular matrix expansion and accumulation (fibronectin and collagen IV levels, etc.). Meanwhile, Sar treatment resulted in decreases in interleukin-18, NLRP3, and activated caspase 1 levels as well as advanced glycation endproducts (AGEs) and their receptor (RAGE) levels in the renal cortex of diabetic rats. However, Sar has no effects on the above indices in the normal rats. Therefore, Sar can markedly ameliorate diabetic nephropathy in rats via inhibition of NLRP3 inflammasome activation and AGEs-RAGE interaction.
Rhizome of Anemarrhena asphodeloides as mediators of the eco-friendly synthesis of silver and gold spherical, face-centred cubic nanocrystals and its anti-migratory and cytotoxic potential in normal and cancer cell lines.[Pubmed: 29595324]
None
Timosaponin A-III inhibits oncogenic phenotype via regulation of PcG protein BMI1 in breast cancer cells.[Pubmed: 29528145]
Polycomb group (PcG) protein BMI1 is an important regulator of oncogenic phenotype and is often overexpressed in several human malignancies including breast cancer. Aberrant expression of BMI1 is associated with metastasis and poor prognosis in cancer patients. At present, therapy reagents that can efficiently inhibit the expression of BMI1 are not very well known. Here, we report that Timosaponin A-III (TA-III), a steroidal saponin obtained from the rhizomes of an herb, Anemarrhena asphodeloides, strongly inhibits expression of BMI1 in breast cancer cells. Treatment of breast cancer cells with TA-III resulted in inhibition of oncogenic phenotypes such as proliferation, migration and invasion, and induction of cellular senescence. Inhibition of these oncogenic phenotypes was accompanied by downregulation of BMI1 expression and histone posttranslational modification activity of PRC1. The mechanistic analysis of TA-III-induced inhibition of oncogenic activity and BMI1 expression suggests that downregulation of c-Myc mediates TA-III effect on BMI1. We further show that exogenous BMI1 overexpression can overcome TA-III-induced inhibition of oncogenic phenotypes. We also show that TA-III induces expression of tumor suppressive miR-200c and miR-141, which are negatively regulated by BMI1. In summary, our data suggest that TA-III is a potent inhibitor of BMI1 and that it can be successfully used to inhibit the growth of tumors where PcG protein BMI1 and PcG activities are upregulated.


