Atractylodes lancea
Atractylodes lancea
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
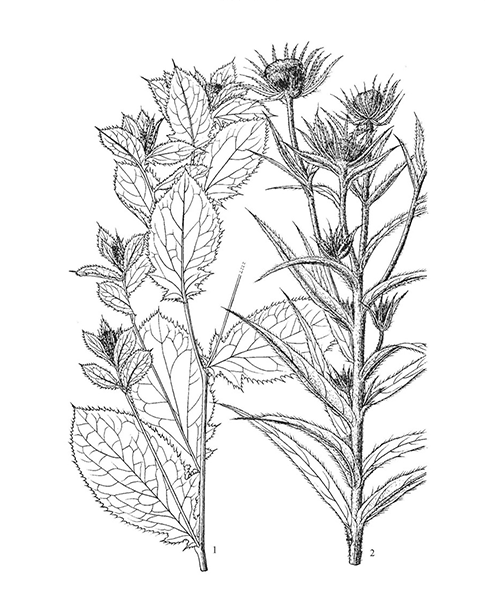
Natural products/compounds from Atractylodes lancea
- Cat.No. Product Name CAS Number COA
-
BCN6059
Syringin118-34-3
Instructions

-
BCN5383
Atractyloside A126054-77-1
Instructions

-
BCN5958
Puerarin3681-99-0
Instructions

-
BCN6294
beta-Eudesmol473-15-4
Instructions

-
BCN5581
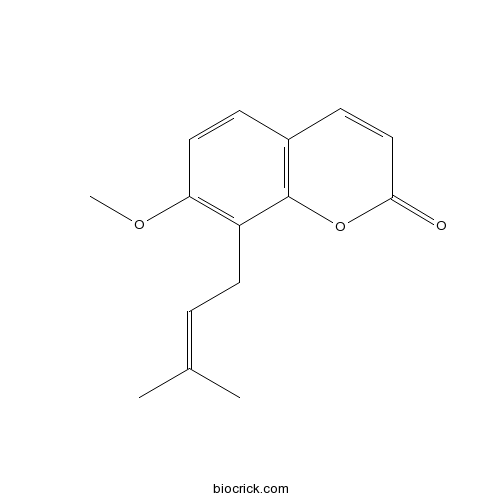
Osthol484-12-8
Instructions
-
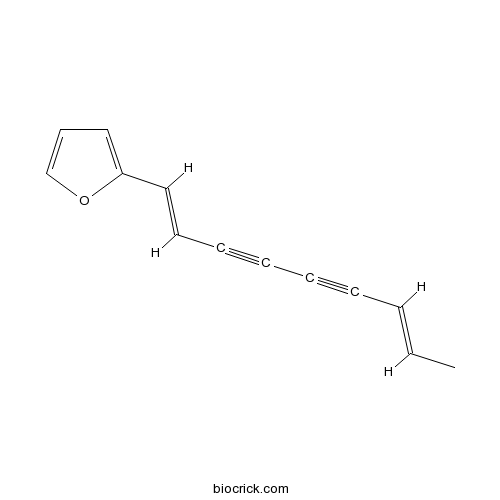
BCN6292
Atractylodin55290-63-6
Instructions
-
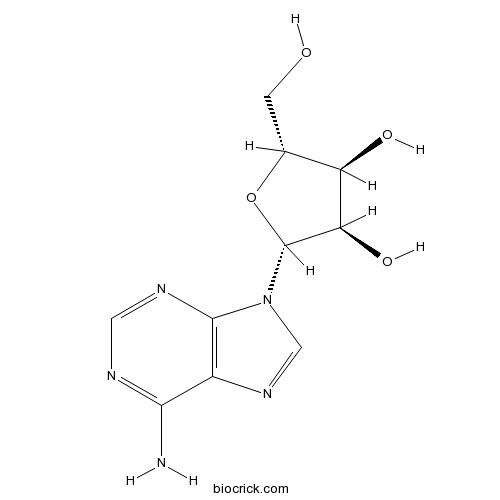
BCN5796
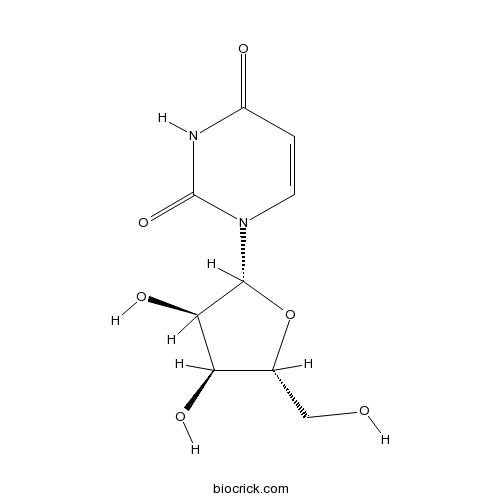
Adenosine58-61-7
Instructions
-
BCN4090
Uridine58-96-8
Instructions
-
BCN1045
Atractylenolide III73030-71-4
Instructions

-
BCN1044
Atractylenolide II73069-14-4
Instructions

Endophytic Pseudomonas induces metabolic flux changes that enhance medicinal sesquiterpenoid accumulation in Atractylodes lancea.[Pubmed: 30081324]
The bacterial endophyte Pseudomonas fluorescens ALEB7B significantly enhances photosynthate accumulations in Atractylodes lancea. These carbohydrates are preferentially used by the host plant to synthesize secondary metabolites, rather than to increase plant biomass accumulation. Mechanisms underlying the allocation of endophyte-increased carbohydrate in different plant metabolic processes are largely unknown. We have studied how P. fluorescens ALEB7B enhances photosynthate accumulation and how bacterial elicitors regulate metabolic flux and increase medicinal sesquiterpenoid formation in A. lancea using the sterile tissue culture plantlets. P. fluorescens ALEB7B enhances plant photosynthate accumulation by synthesizing and secreting indole-3-acetic acid, which has been demonstrated using high-performance liquid chromatography analysis. The increased endogenous indole-3-acetic acid promotes plant root development and then assimilation. Increased carbohydrates provide the material basis for the formations of terpenoid hydrocarbon scaffolds, which has been proved using gas chromatography analysis. Further, protein and polysaccharide elicitors secreted by P. fluorescens ALEB7B have been separated and purified from the bacterial fermentation broth, which have been applied to A. lancea plantlets. Both elicitors can stimulate the conversions of terpenoid hydrocarbon scaffolds to oxygenous sesquiterpenoids, the active medicinal ingredients in A. lancea, by triggering the oxidative burst in planta. Moreover, this study separates an ABC transporter substrate-binding protein from protein elicitors secreted by P. fluorescens ALEB7B with an ÄKTA Prime Plus Purifier System and firstly shows that this protein is essential to induce oxygenous sesquiterpenoid accumulation in A. lancea. This study provides new perspectives for mechanisms of medicinal oxygenous terpenoid synthesis, which has important reference values to the cultivation of medicinal plants that have terpenoids as their active ingredients, such as Artemisia annua and Taxus chinensis.
Two new thiophene polyacetylene glycosides from Atractylodes lancea.[Pubmed: 29614875]
Phytochemical investigation on the rhizomes of Atractylodes lancea led to the isolation of two new thiophene polyacetylene glycosides (1 and 2) and six known compounds (3-8). Their structures were elucidated based on the extensive spectroscopic data (UV, IR, 1D and 2D NMR, and HRESIMS). The absolute configurations of new compounds were established by calculated and experimental circular dichroism. All the compounds were assessed on the lipopolysaccharide-induced NO production in BV2 cells and compounds 3, 7, and 8 showed moderate inhibitory activities.


