Chloranthus japonicus
Chloranthus japonicus
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Chloranthus japonicus
- Cat.No. Product Name CAS Number COA
-
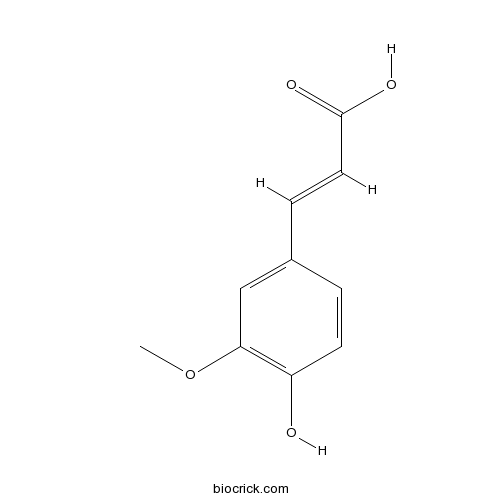
BCN5948
Ferulic acid1135-24-6
Instructions
-
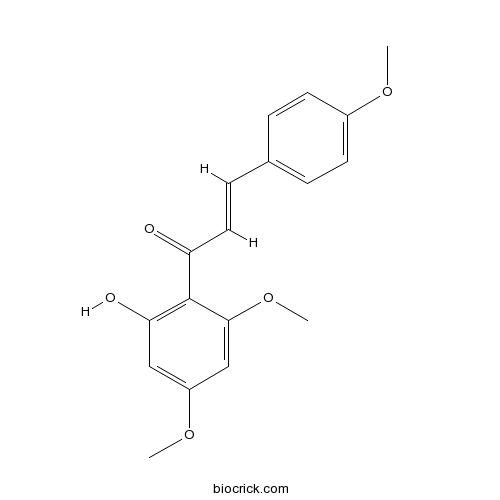
BCN9056
Flavokawain A37951-13-6
Instructions
-
BCN1045
Atractylenolide III73030-71-4
Instructions

A new lindenane-type sesquiterpenoid lactone from Chloranthus japonicus.[Pubmed: 29338351]
None
Chlorajaponols A-F, sesquiterpenoids from Chloranthus japonicus and their in vitro anti-inflammatory and anti-tumor activities.[Pubmed: 28408269]
None
Modulation of glucose metabolism by a natural compound from Chloranthus japonicus via activation of AMP-activated protein kinase.[Pubmed: 28396610]
AMP-activated protein kinase (AMPK) is a key sensor and regulator of glucose metabolism. Here, we demonstrated that shizukaol F, a natural compound isolated from Chloranthus japonicus, can activate AMPK and modulate glucose metabolism both in vitro and in vivo. Shizukaol F increased glucose uptake in differentiated C2C12 myotubes by stimulating glucose transporter-4 (GLUT-4) membraned translocation. Treatment of primary mouse hepatocytes with shizukaol F decreased the expression of phosphoenolpyruvate carboxykinase 2 (PEPCK), glucose-6-phosphatase (G6Pase) and suppressed hepatic gluconeogenesis. Meanwhile, a single oral dose of shizukaol F reduced gluconeogenesis in C57BL/6 J mice. Further studies indicated that shizukaol F modulates glucose metabolism mainly by AMPKa phosphorylation activity. In addition, we also found that shizukaol F depolarizes the mitochondrial membrane and inhibits respiratory complex I, which may result in AMPK activation. Our results highlight the potential value of shizukaol F as a possible treatment of metabolic syndrome.
Hot off the press.[Pubmed: 28079908]
A personal selection of 32 recent papers is presented covering various aspects of current developments in bioorganic chemistry and novel natural products such as hitorin A from Chloranthus japonicus.
Lindenane sesquiterpenoid dimers from Chloranthus japonicus inhibit HIV-1 and HCV replication.[Pubmed: 27705755]
None
Chlojaponilactone B from Chloranthus japonicus: Suppression of Inflammatory Responses via Inhibition of the NF-κB Signaling Pathway.[Pubmed: 27588583]
Bioassay-guided fractionation of an ethanolic extract of Chloranthus japonicus led to the isolation of the known lindenane-type sesquiterpenoid chlojaponilactone B (1). This compound exhibited pronounced inhibition of nitric oxide (NO) production in lipopolysaccharide (LPS)-induced RAW 264.7 macrophages. Further anti-inflammatory assays showed that 1 suppressed the levels of some key inflammation mediators, such as iNOS, TNF-α, and IL-6, in a dose-dependent manner, and reduced the ear thickness and neutrophil infiltration in 12-O-tetradecanoylphorbol-13-acetate (TPA)-stimulated mice. A mechanistic study revealed that compound 1 exerted its anti-inflammatory effects via the suppression of the NF-κB signaling pathway, which inhibited NF-κB-dependent transcriptional activity, IκBα phosphorylation, and p65 nuclear translocation. In contrast, chlojaponilactone B (1) was found to exert little influence on the MAPK signaling pathway.
Two new sesquiterpenes from Chloranthus japonicus Sieb.[Pubmed: 27399937]
Two new sesquiterpenes, namely, 1β,10β-dihydroxy-eremophil-7(11), 8-dien-12,8-olide (1) and 8,12-epoxy-1β-hydroxyeudesm-3,7,11-trien-9-one (2), together with three known sesquiterpenoids, shizukolidol (3), 4α-hydroxy-5α(H)-8β-methoxy-eudesm-7(11)-en-12,8-olide (4), and neolitacumone B (5), and two known monoterpenes, (3R,4S,6R)-p-menth-1-en-3,6-diol (6) and (R)-p-menth-1-en-4,7-diol (7), were isolated from the whole plant of Chloranthus japonicus Sieb. Their structures were elucidated on the basis of spectroscopic data analysis and comparison with those of related known compounds. Compounds 4-7 were isolated from this plant for the first time.
Natural nitric oxide (NO) inhibitors from Chloranthus japonicus.[Pubmed: 27177824]
Eight new lindenane sesquiterpenoid dimers, chlojapolides A-H (1-8), along with 11 known analogues were isolated from the whole plant of Chloranthus japonicus. Their structures including absolute configurations were elucidated by spectral and chemical methods. All the compounds were examined for their inhibitory effects on the nitric oxide (NO) production induced by lipopolysaccharide (LPS) in RAW 264.7 macrophages, and compounds 1, 11, 13, and 17 exhibited pronounced inhibition with IC50 values in the range of 6.91-15.75μM, being more active than the positive control, quercetin (IC50=15.90μM).


