Clinopodium chinense
Clinopodium chinense
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.

Natural products/compounds from Clinopodium chinense
- Cat.No. Product Name CAS Number COA
-
BCN6300
Narirutin14259-46-2
Instructions

-
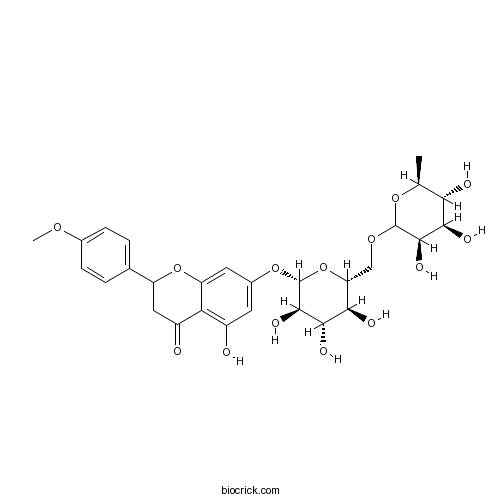
BCN3327
Didymin14259-47-3
Instructions
-
BCN2842
Buddlejasaponin IVb152580-79-5
Instructions

-
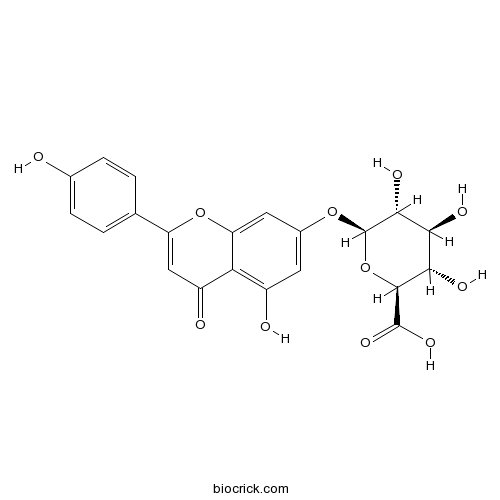
BCN5326
Apigenin-7-glucuronide29741-09-1
Instructions
-
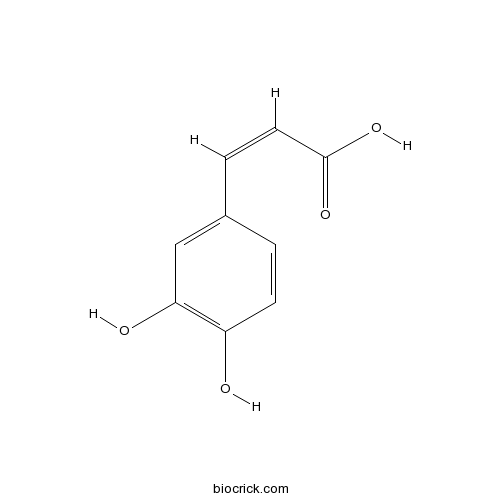
BCN5979
Caffeic acid331-39-5
Instructions
-
BCN5554
Linarin480-36-4
Instructions

-
BCN5559
Isosakuranetin480-43-3
Instructions

-
BCN5658
Apigenin520-36-5
Instructions

-
BCN1209
Eriodictyol552-58-9
Instructions

Two new flavonoid-triterpene saponin meroterpenoids from Clinopodium chinense and their protective effects against anoxia/reoxygenation-induced apoptosis in H9c2 cells.[Pubmed: 29782903]
Two new flavonoid-triterpene saponin meroterpenoids, clinoposides G (1) and H (2) were isolated from the aerial parts of Clinopodium chinense (Benth.) O. Kuntze. Their structures were elucidated through spectroscopic and electronic circular dichroism (ECD) analyses. Compounds 1 and 2 were evaluated for their protective effects against anoxia/reoxygenation(A/R)-induced injury in H9c2 cells. A/R treatment severely injured the H9c2 cells, which was accompanied by apoptosis. Both 1 and 2 pretreatment significantly inhibited cell injury and apoptosis, improved mitochondrial membrane potential, increased activities of antioxidant enzymes, and reduced the levels of the inflammatory cytokines. In addition, the presence of 1 and 2 significantly decreased the protein level of p65 and increased the level of Nrf2 in cell nucleus. Unique chemical structure and good biological activity of 1 and 2 elucidated the potential of meroterpenoids as a promising reagent for treating heart disease.
Protective effects of total flavonoids from Clinopodium chinense (Benth.) O. Ktze on myocardial injury in vivo and in vitro via regulation of Akt/Nrf2/HO-1 pathway.[Pubmed: 29496179]
Clinopodium chinense (Benth.) O. Ktze is a traditional Chinese herbal medicine, which comprises the plant's total flavonoids. TFCC plays an important role in the treatment of cardiovascular disease.
Acute and a 28-day repeated-dose toxicity study of total flavonoids from Clinopodium chinense (Benth.) O. Ktze in mice and rats.[Pubmed: 29107008]
Clinopodium chinense (Benth.) O. Ktze (Labiatae), known as 'Duanxueliu' in the Chinese Pharmacopoeia, has been widely used as a traditional Chinese medicine for the treatment of hemorrhagic disease. Total flavonoids from Clinopodium chinense (Benth.) O. Ktze (TFCC), the most active ingredient, possess a variety of properties, such as antioxygenation. Until now, evidence-based toxicity data on TFCC has been limited. This study evaluated the acute (in mice and rat) and the 28-day repeated-dose (in rat) toxicity study of TFCC, respectively. In acute study, oral administration of TFCC to rats and mice did not induce toxicity or mortality up to the maximum doses of 4000 and 5000 mg/kg, respectively. In subacute toxicity study, we administered TFCC at daily doses of 70, 210, and 630 mg/kg for 4 consecutive weeks to rats via gavage. We observed no changes in food consumption, water intake, body weight, chemistry and hematological parameters, organ weight, gross pathology or histopathology. No animals from any group died. These findings indicate that TFCC is relatively nontoxic, and provide practical guidance for selecting a safe dose for further investigation of TFCC in animal studies or clinical trials.
Triterpenoid saponins from Clinopodium chinense (Benth.) O. Kuntze and their biological activity.[Pubmed: 28895057]
None
[Studies on chemical constituents of Clinopodium chinense].[Pubmed: 28840692]
Twenty-eight compounds were isolated and purified from Clinopodium chinense by Sephedax LH-20, ODS, MCI and preparative HPLC. Their structures were identified as apigenin (1), apigenin-7-O-β-D-glucopyranoside (2), apigenin-7-O-β-D-glucuronopyranoside (3), thellungianol (4), apigenin-7-O-β-D-rutinoside (5), luteolin (6), luteolin-4'-O-β-D-glucopyranoside (7), apigenin-7-O-β-D-pyranglycuronate butyl ester (8), luteolin-7-O-β-D-rutinoside (9), luteolin-7-O-β-D-noehesperidoside (10), acacetin (11), acacetin-7-O-β-D-glucuronopyranoside (12), buddleoside (13), naringenin (14), pruning (15), nairutin (16), isosakuranetin (17), isosakuranin (18), didymin (19), hesperidin (20), kaempferol (21), quercetin (22), kaempferol-3-O-α-L-rahmnoside (23), p-hydroxycinnamic acid (24), caffeic acid (25), cis-3-[2-[1-(3,4-dihydroxy-phenyl)-1 -hydroxymethyl]-1,3-ben-zodioxol-5-yl]-(E)-2-propenoic acid (26), mesaconic acid (27), gentisic acid 5-O-β-D-(6'-salicylyl)-glucopyranoside (28). Among them, compounds 7, 9-10, 12, 23, 26-28 were isolated from the Clinopodium for the first time. The protective effects of compounds 1-6, 8-17 and 19 against H2O2-induced H9c2 cardiomyocyte injury were tested, compounds 15 exhibited significantly protective effects. Compared with the cell viability of (62.12±6.18)% in the model, pruning exhibited viabilities of (84.25±7.36)% at 25.0 mg•L⁻¹, respectively, using quercetin as a positive control [cell viability of (84.55±8.26)%, 20 mg•L⁻¹].
Phenolic Compounds from Clinopodium chinense (Benth.) O. Kuntze and Their Inhibitory Effects on α-Glucosidase and Vascular Endothelial Cells Injury.[Pubmed: 27088891]
Following an in vitro bioactivity-guided fractionation procedure, 14 compounds including eight flavonoids and six phenylpropanoids were isolated and identified from the AcOEt fraction of Clinopodium chinense (Benth.) O. Kuntze. All constituents were tested for α-glucosidase and high glucose-induced injury in human umbilical vein endothelial cells (HUVECs) inhibitory activities. All constituents exhibited varying degrees α-glucosidase inhibitory activity and protective activity on HUVECs. Among them, luteolin (2), eriodictyol (5), ethyl rosmarinate (13), and clinopodic acids B (14) were proved to be potent α-glucosidase inhibitors with IC50 value ranging from 0.6 to 2.0 μm. Additionally, luteolin (2), naringenin (4), eriodictyol (5), ethyl (2R)-3-(3, 4-dihydroxyphenyl)-2-hydroxypropanate (9), caffeic acid (11), ethyl rosmarinate (13), and clinopodic acids B (14) significantly ameliorate HUVECs injury induced by high glucose with an approximate EC50 value of 3 - 36 μm. These results suggest that the 14 bioactive constituents were responsible for hypoglycemic and protective vascular endothelium effect of C. chinense (Benth.) O. Kuntze and their structure-activity relationship was also analyzed briefly. Eriodictyol, luteolin, ethyl rosmarinate, and clinopodic acids B were the potential lead compounds of antidiabetic drugs.
Two new abietane diterpenoid glycosides from Clinopodium chinense.[Pubmed: 26551245]
Two new abietane diterpenoid glycosides, named clinopoditerpenes B (1) and C (2), were isolated from Clinopodium chinese. The structures of the new compounds were determined on the basis of extensive spectral analysis. Compound 1 exhibited cardioprotective effect against H2O2-induced apoptosis in H9c2 cells.
Chemical Composition and Insecticidal Activities of the Essential Oil of Clinopodium chinense (Benth.) Kuntze Aerial Parts against Liposcelis bostrychophila Badonnel.[Pubmed: 26408136]
Water-distilled essential oil from Clinopodium chinense (Labiatae) aerial parts at the flowering stage was analyzed by gas chromatography-mass spectrometry. Thirty-five compounds, accounting for 99.18% of the total oil, were identified, and the main components of the essential oil of C. chinense were spathulenol (18.54%), piperitone (18.9%), caryophyllene (12.04%), and bornyl acetate (8.14%). Based on bioactivity-directed fractionation, bornyl acetate, caryophyllene, and piperitone were identified from the essential oil. The essential oil possessed fumigant toxicity against booklice (Liposcelis bostrychophila) with a 50% lethal concentration (LC50) value of 423.39 μg/liter, while the isolated constituents, bornyl acetate and piperitone, had LC50 values of 351.69 and 311.12 μg/liter against booklice, respectively. The essential oil also exhibited contact toxicity against L. bostrychophila with an LC50 value of 215.25 μg/cm(2). Bornyl acetate, caryophyllene, and piperitone exhibited acute toxicity against booklice with LC50 values of 321.42, 275.00, and 139.74 μg/cm(2), respectively. The results indicated that the essential oil and its isolated constituents have potential for development into natural insecticides or fumigants for control of insects in stored grains.
Total Flavonoids from Clinopodium chinense (Benth.) O. Ktze Protect against Doxorubicin-Induced Cardiotoxicity In Vitro and In Vivo.[Pubmed: 25784945]
Doxorubicin has cardiotoxic effects that limit its clinical benefit in cancer patients. This study aims to investigate the protective effects of the total flavonoids from Clinopodium chinense (Benth.) O. Ktze (TFCC) against doxorubicin- (DOX-) induced cardiotoxicity. Male rats were intraperitoneally injected with a single dose of DOX (3 mg/kg) every 2 days for three injections. Heart samples were collected 2 weeks after the last DOX dose and then analyzed. DOX delayed body and heart growth and caused cardiac tissue injury, oxidative stress, apoptotic damage, mitochondrial dysfunction, and Bcl-2 expression disturbance. Similar experiments in H9C2 cardiomyocytes showed that doxorubicin reduced cell viability, increased ROS generation and DNA fragmentation, disrupted mitochondrial membrane potential, and induced apoptotic cell death. However, TFCC pretreatment suppressed all of these adverse effects of doxorubicin. Signal transduction studies indicated that TFCC suppressed DOX-induced overexpression of p53 and phosphorylation of JNK, p38, and ERK. Studies with LY294002 (a PI3K/AKT inhibitor) demonstrated that the mechanism of TFCC-induced cardioprotection also involves activation of PI3K/AKT. These findings indicated the potential clinical application of TFCC in preventing DOX-induced cardiac oxidative stress.


