Curcuma phaeocaulis
Curcuma phaeocaulis
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
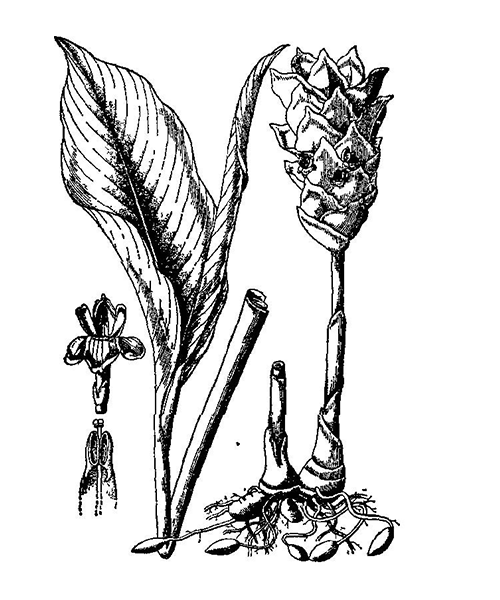
Natural products/compounds from Curcuma phaeocaulis
- Cat.No. Product Name CAS Number COA
-
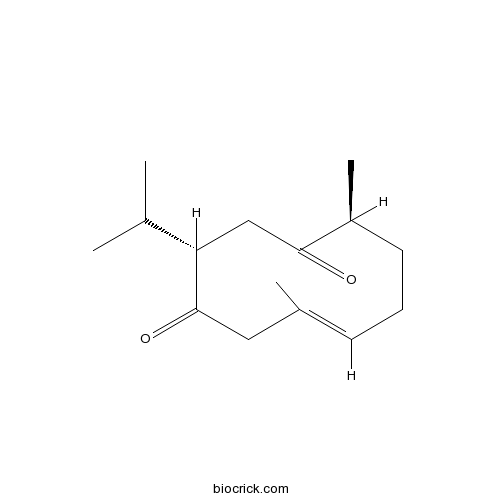
BCN5936
Curdione13657-68-6
Instructions
-
BCN3522
Curcumenol19431-84-6
Instructions

-
BCN3526
Isocurcumenol24063-71-6
Instructions

-
BCN3010
Furanodienone24268-41-5
Instructions

-
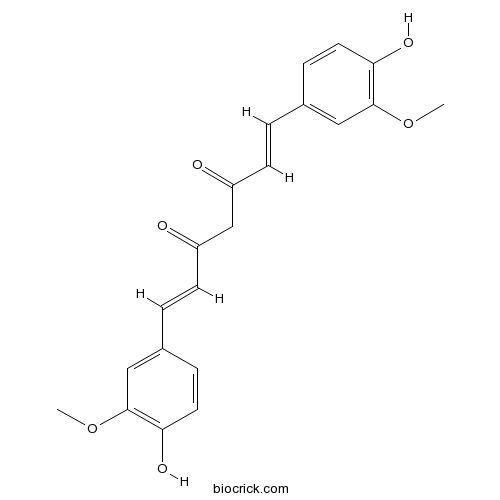
BCN5504
Curcumin458-37-7
Instructions
-
BCN5976
Curcumol4871-97-0
Instructions

-
BCN4981
Germacrone6902-91-6
Instructions

Diarylheptanoids from Curcuma phaeocaulis Suppress IL-6-Induced STAT3 Activation.[Pubmed: 30096715]
None
The Effects of Curcumae Longae Radix, Curcuma phaeocaulis Radix and Their Processed Products on Epo/EpoR Pathway and CD62p.[Pubmed: 30038572]
None
Protein network module-based identification of key pharmacological pathways of Curcuma phaeocaulis Val. acting on hepatitis.[Pubmed: 29526702]
Curcuma phaeocaulis Val. (CP), as the vital medicines for blood-breaking and disorder-eliminating, has been widely used for hepatitis with good curative effects. Owing to the complexity of traditional Chinese medicine, the pharmacological mechanism of CP remains unclear. To solve this problem, a protein network module-based approach was proposed in this study.
(+)/(-)-Phaeocaulin A-D, four pairs of new enantiomeric germacrane-type sesquiterpenes from Curcuma phaeocaulis as natural nitric oxide inhibitors.[Pubmed: 28272397]
Germacrane-type sesquiterpenes, with a flexible 10-membered ring unit as the structural and conformational features, play a central role in the biosynthesis and synthesis of other sesquiterpenes. In this report, two pairs of new sesquiterpene alkaloids, (+)/(-)-phaeocaulin A [(+)-1/(-)-1] and B [(+)-2/(-)-2], and two pairs of new sesquiterpenes, (+)/(-)-phaeocaulin C [(+)-3/(-)-3] and D [(+)-4/(-)-4], along with one related known analog (5), were isolated from the rhizomes of Curcuma phaeocaulis. The absolute configurations of (+)-1/(-)-1, (+)-2/(-)-2, (+)-3/(-)-3 and (+)-4/(-)-4 were unambiguously determined by analysis of single-crystal X-ray diffractions and quantum chemical electronic circular dichroism (ECD) method. It is noteworthy that (+)/(-)-phaeocaulin A [(+)-1/(-)-1] and B [(+)-2/(-)-2] are two pairs of rare N-containing germacrane-type sesquiterpenes. A possible biogenetic pathway for 1-5 was postulated. All of the isolated compounds were tested for their inhibitory activity against LPS-induced nitric oxide production in RAW 264.7 macrophages.
An investigation of the developmental neurotoxic potential of curcumol in PC12 cells.[Pubmed: 27819177]
Curcuma phaeocaulis Val. is a Chinese medicinal herb that is contraindicated during pregnancy for over a thousand years in China. The aims of the present study were to evaluate the effect of curcumol (one of the major components of C. phaeocaulis Val.) on neurite outgrowth and characterize the signal transduction pathways in PC12 cells. Curcumol significantly inhibited neurite outgrowth and cell proliferation, but did not cause cell death at a concentration of 450 μM in differentiated PC12 cells. In addition, curcumol evoked oxidative stress and it was indicated by an elevation in reactive oxygen species (ROS) and lipid peroxidation (LPO). Although PC12 cells exhibited inhibition of the differentiation into the acetylcholine (ACh) phenotype following 450 μM curcumol exposure, there was no significant alteration in net shift toward the ACh phenotype or tyrosine hydroxylase (TH) phenotype was observed. Neural cell adhesion molecule (NCAM)/focal adhesion kinase (FAK) but not extracellular signal-regulated kinases 1 and 2 (ERK1/2) signaling was repressed by curcumol exposure in differentiated PC12 cells. Curcumol does not affect calpain activity and nuclear factor-κB (NF-κB) DNA-binding activity. These findings suggest that curcumol might be a developmental neurotoxicant and NCAM/FAK signaling pathway may play an important role in curcumol-evoked inhibition of neurite outgrowth.
Antiproliferative activity of Curcuma phaeocaulis Valeton extract using ultrasonic assistance and response surface methodology.[Pubmed: 26914409]
None
Sesquiterpenoids from the Rhizomes of Curcuma phaeocaulis and Their Inhibitory Effects on LPS-Induced TLR4 Activation.[Pubmed: 27373668]
Two new guaiane-type (2, 6) and one new furanogermacrane-type (11) sesquiterpenoids have been isolated along with twelve known compounds from an EtOAc-soluble extract of Curcuma phaeocaulis rhizomes. The structures of the isolated compounds were elucidated using a combination of NMR, MS, and circular dichroism (CD) spectra. The inhibitory effects of each compound on lipopolysaccharide (LPS)-induced Toll-like receptor 4 (TLR4) activation in THP-1-Blue cells were assessed, and compound 4 showed more potent inhibitory activity against LPS-stimulated TLR4 activation.
[Analysis on transfer rate change of heavy metal in decoction and precipitation of Curcuma phaeocaulis before and after processing with vinegar based on ICP-MS].[Pubmed: 28845642]
Chinese herbs are mostly used to make decoction, which would form precipitation after standing for cooling and abandoned by patients. Processing with vinegar can change the property of the herbal pieces, such as the transfer rate of heavy metal into decoction. To analyze the transfer rate change of heavy metal in the decoction and precipitation of Curcuma phaeocaulis before and after processing with vinegar, inductively coupled plasma mass spectrometry (ICP-MS) was used to establish the determination method on five heavy metals in C. phaeocaulis, including Copper (Cu), arsenic (As), chromium (Cd), lead (Pb), mercury (Hg), using microwave to digest the samples, indium (In) as the internal standard, and national level standard material tea leaves GBW10016 (GSB-7 tea) as the quality control standard material. Then, the content of five heavy metals in the herbal pieces, decoction and five heavy metals of 6 representative batches of C. phaeocaulis and their vinegar-processing products was determinated. After computation, the transfer rates of heavy metals in the decoction and precipitation of C. phaeocaulis Val. before and after the processing with vinegar were obtained. The results showed that, after the processing with vinegar, total transfer rate of Pb and Hg was decreased significantly; total transfer rate of Cd and Cu was slightly decreased; total transfer rate of As was slightly increased, however heavy metals in all the precipitation were decreased. The results indicated that processing with vinegar had certain influence on heavy metal transfer rate, with certain synergistic and attenuated effect.


