Cynanchum paniculatum
Cynanchum paniculatum
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
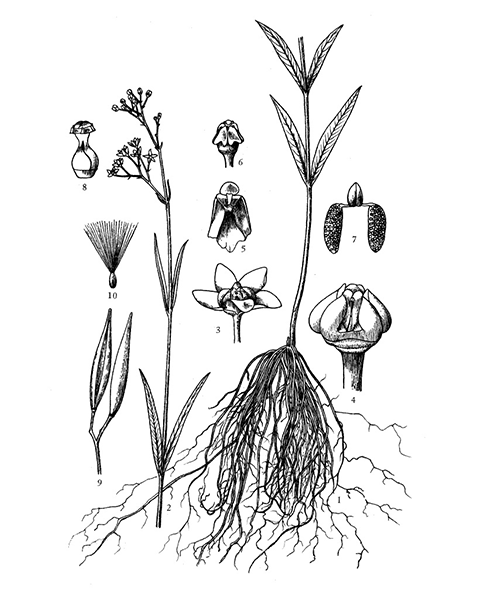
Natural products/compounds from Cynanchum paniculatum
- Cat.No. Product Name CAS Number COA
-
BCN6186
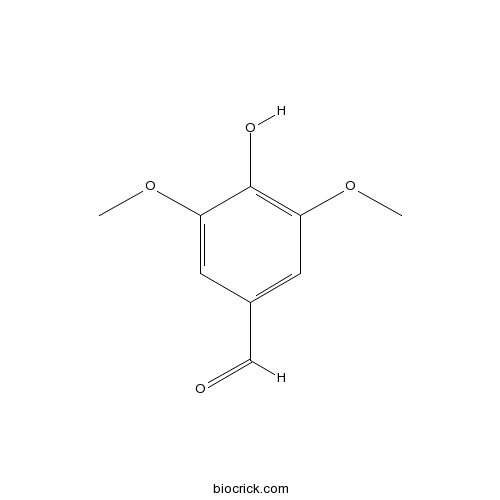
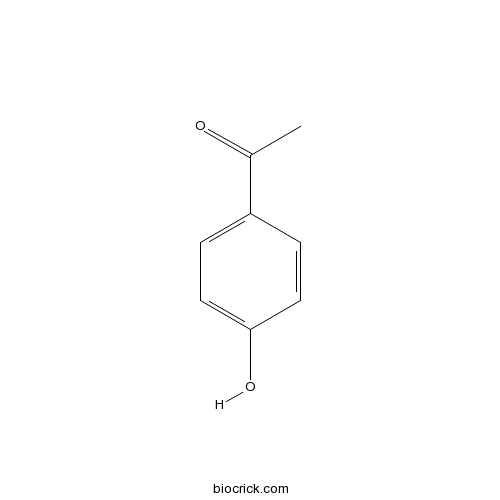
4-Hydroxy-3,5-dimethoxybenzaldehyde134-96-3
Instructions
-
BCN3837
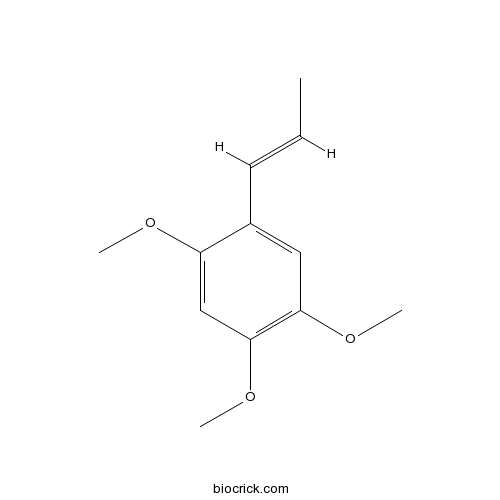
alpha-Asarone2883-98-9
Instructions
-
BCN5738
Paeonol552-41-0
Instructions

-
BCN4090
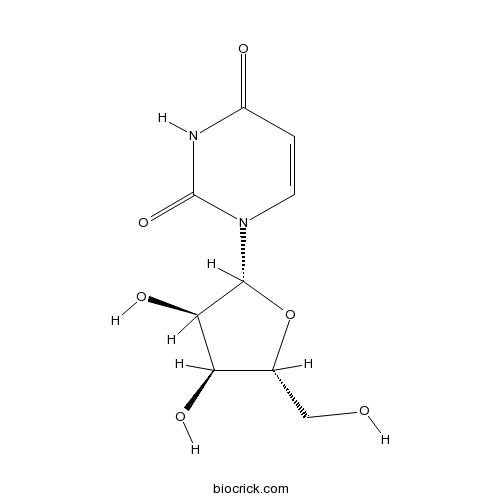
Uridine58-96-8
Instructions
-
BCN2805
Paeonolide72520-92-4
Instructions

-
BCN4544
4'-Hydroxyacetophenone99-93-4
Instructions
Anti-Deinagkistrodon acutus venom properties of ethanolic root extract from Cynanchum paniculatum (Bunge) kitag and its GC-MS analysis.[Pubmed: 30033377]
Cynanchum paniculatum (Bunge) Kitag known as a popular Chinese herbal medicine has been used for a long time to treat a wide variety of diseases including snakebites. However, there is scarce information on the antiophidian potential of this plant.
Activity of two extracts of Cynanchum paniculatum against Ichthyophthirius multifiliis theronts and tomonts.[Pubmed: 27928976]
The present study aims to evaluate the antiparasitic activity of active components from Cynanchum paniculatum against Ichthyophthirius multifiliis. The antiparasitic activities of two bioassay-guided fractionationated compounds from C. paniculatum identified as Cynatratoside-A and Cynanversicoside C, by comparing spectral data (NMR and ESI-MS) with literature values, were evaluated by in vitro assay. These showed that both could kill theronts of I. multifiliis at a concentration of 10·0 mg L-1, with the median effective concentration (EC50) values of 4·6 mg L-1 and 5·2 mg L-1 for Cynatratoside-A and Cynanversicoside C, respectively. Encysted tomonts were killed at concentrations of 8·0 mg L-1 with both compounds. In vivo experiments demonstrated that fish treated with both compounds at 15·0 mg L-1 carried significantly fewer parasites than controls (P < 0·05). There were no mortalities among treated fish group compared with 75% mortality of untreated fish. Cynatratoside-A and Cynanversicoside C are therefore potential candidate drugs for use against I. multifiliis.
C21 steroidal glycosides from the roots of Cynanchum paniculatum.[Pubmed: 27380712]
As a part of our continuing research for bioactive constituents from Cynanchum plants, four new C21 steroidal glycosides, cynapanoside D-G (1-4), together with six known compounds (5-10) were isolated from the roots of Cynanchum paniculatum (Bge.) Kitag. Their structures were elucidated on the basis of 1D- and 2D-NMR spectroscopic data as well as HR-ESI-MS analysis. Compound 8 exhibited potent inhibitory activities against HL-60, HT-29, PC-3 and MCF-7 cell lines with IC50 values of 8.3, 7.5, 34.3 and 19.4μM, respectively and compounds 1-4 and 9 displayed moderate cytotoxicity against the four cell lines. The in vitro antioxidant activities of compounds 1-4, 8 and 9 were assayed by DPPH radical scavenging activity. Antibacterial and antifungal activities of compounds 1-4, 8 and 9 were also tested.
Evaluation of medicated feeds with antiparasitical and immune-enhanced Chinese herbal medicines against Ichthyophthirius multifiliis in grass carp (Ctenopharyngodon idellus).[Pubmed: 27003405]
Since malachite green was banned for using in food fish due to its carcinogenic and teratogenic effects on human, the search of alternative drug to treat Ichthyophthirius multifiliis becomes urgent. This study aimed to (1) evaluate the ethanol extracts of medicinal plants Cynanchum atratum, Zingiber officinale, Cynanchum paniculatum, immunostimulant (A), and immunostimulant (B) for their efficacy against I. multifiliis, and (2) determine effects of medicated feeds with C. atratum, Z. officinale, C. paniculatum, and immunostimulant (A) to treat I. multifiliis in grass carp. The results in this study showed that the minimum concentrations of C. atratum, Z. officinale, and C. paniculatum extracts for killing all theronts were 16, 8, and 16 mg/L, respectively. In vivo experiments, fish fed with medicated feeds of C. atratum for 10 days, or Z. officinale for 3 days, or combination of three plants for 10 days resulted in a significant reduction in the I. multifiliis infective intensity on grass carp after theronts exposure. Grass carp fed with medicated feeds of immunostimulant (A) for 21 days showed no infection and 100 % of survival 15 days post theronts exposure. Therefore, immunostimulant (A) is a promising feed supplement to treated I. multifiliis with good antiparasitic efficacy.
Xiangqing anodyne spray (XQAS): a combination of ethanol extracts of Cynanchum paniculatum and Illicium henryi for treating soft-tissue injury.[Pubmed: 26550185]
To compare the pharmacodynamic effects of an anodyne spray (XQAS) containing extracts of two herbs, Cynanchum paniculatum (CP) and Illicium henryi (IH), with those of spray containing the vehicle alone, CP alone (CPS) or IH alone (IHS), when applied topically acute soft tissue injury (STI) in an animal model.
Two new 13,14:14,15-disecopregnane-type compounds from the roots of Cynanchum paniculatum.[Pubmed: 26465069]
Two new 13,14:14,15-disecopregnane-type compounds glaucogenin F (1), glaucogenin F 3-O-β-D-oleandropyranoside (2), together with three known compounds cynapanoside A (3), cynatratoside A (4), and neocynapanogenin F 3-O-β-D-oleandropyranoside (5) were isolated from the 95% ethanol extract of the roots of Cynanchum paniculatum. The structures of new compounds were elucidated on the basis of extensive spectroscopic analyses, including 1D and 2D NMR, HRESIMS and NOESY.


