Engelhardia roxburghiana
Engelhardia roxburghiana
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Engelhardia roxburghiana
- Cat.No. Product Name CAS Number COA
-
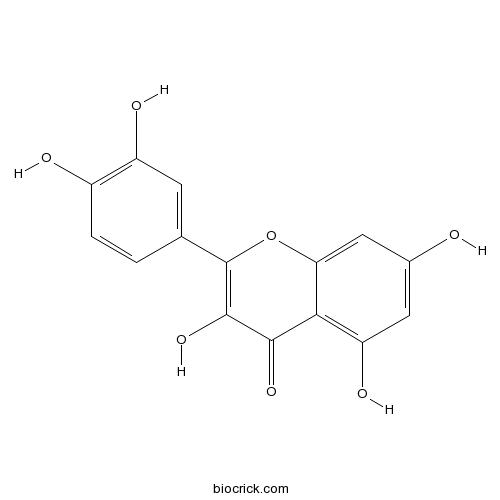
BCN6049
Quercetin117-39-5
Instructions
-
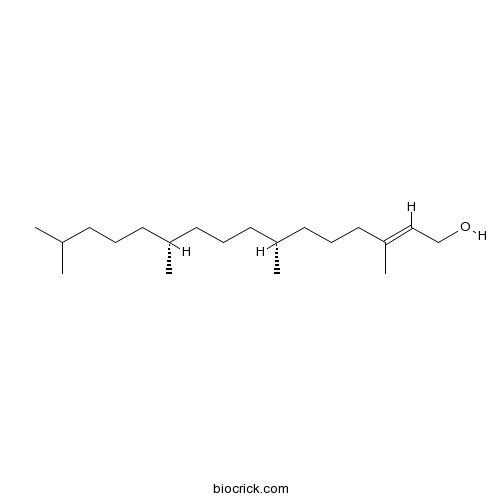
BCN1673
Phytol150-86-7
Instructions
-
BCN5550
Taxifolin480-18-2
Instructions

-
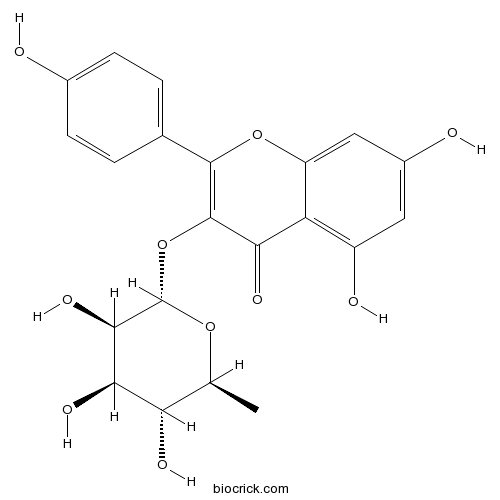
BCN5573
Afzelin482-39-3
Instructions
-
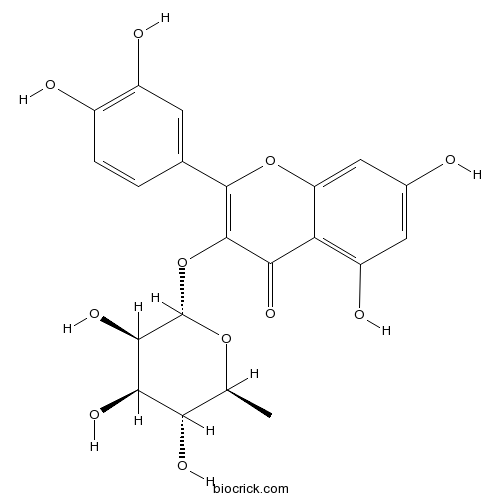
BCN5665
Quercitrin522-12-3
Instructions
-
BCN8853
Neoastilbin54081-47-9
Instructions

-
BCN5719
Isoastilbin54081-48-0
Instructions
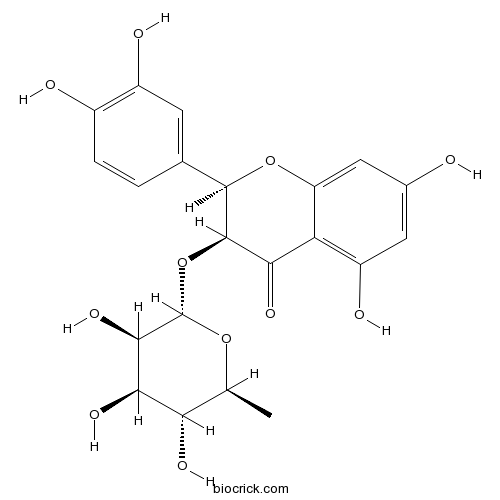
-
BCN6532
Neoisoastilbin54141-72-9
Instructions

-
BCN5772
Engeletin572-31-6
Instructions

Phenology-dependent variation in the non-structural carbohydrates of broadleaf evergreen species plays an important role in determining tolerance to defoliation (or herbivory).[Pubmed: 28860453]
Two broadleaf evergreen canopy species (Schima superba and Engelhardia roxburghiana) with different phenologies in a subtropical region of southern China were used to determine the influence of leaf phenology on the impact of an insect pest attack. S. superba regenerates its leaves in February, while E. roxburghiana regenerates its leaves in May. The moth Thalassodes quadraria attacked the two broadleaf evergreen species in March to April, and the newly produced leaves were removed for S. superba but not for E. roxburghiana. The young trees were artificially defoliated to imitate an insect pest attack during March 2014. Nonstructural carbohydrate (NSC) and growth measurements and a retrospective analysis based on the radial growth of mature trees were conducted in January 2015. The results showed that NSC concentrations decreased in S. superba during canopy rebuilding, and the subsequent defoliation severely inhibited leaf and shoot growth, prevented NSC restoration in roots and stem xylem, and caused high mortality. The insect outbreaks reduced the radial growth of S. superba. In contrast, E. roxburghiana experienced less growth retardation, lower mortality, and normal radial growth. Thus, taking phenology-dependent variation in NSCs into consideration, defoliation and insect pest outbreaks more negatively impacted S. superba than E. roxburghiana.
Separating four diastereomeric pairs of dihydroflavonol glycosides from Engelhardia roxburghiana using high performance counter-current chromatography.[Pubmed: 25620740]
Four pairs of diastereomers were successfully isolated and separated from the water extract of Engelhardia roxburghiana by high performance counter-current chromatography (HPCCC) using a two-step procedure. The diastereomers were initially separated by a two-phase solvent system composed of n-hexane-n-butanol-0.1% trifluoroacetic acid (1:2:3, v/v/v) and followed by the same solvent system using hydroxypropyl-β-cyclodextrin (HP-β-CD) as an additive. The chromatographic conditions, elution mode, and concentrations of the additive were refined. The two-step HPCCC isolation yielded 43.7mg (2S, 3S)-astilbin, 27.6mg (2R, 3R)-astilbin, 5.9mg (2S, 3R)-astilbin, 4.8mg (2R, 3S)-astilbin, 6.9mg (2S, 3S)-engelitin, 3.1mg (2R, 3R)-engelitin, 8.2mg (2S, 3R)-engelitin, and 6.0mg (2R, 3S)-engelitin from 384mg crude extract in four runs with purities of 99.3%, 96.2%, 99.8%, 99.9%, 97.0%, 96.5%, 96.1%, and 96.8%, respectively. The present study revealed that HP-β-CD can be used as an additive in HPCCC to effectively improve the resolution of the diastereomers. The established HPCCC method may serve as an approach to obtain high purity diastereomers on a large scale.
Utility of hesperidinase for food function research: enzymatic digestion of botanical extracts alters cellular antioxidant capacities and anti-inflammatory properties.[Pubmed: 25093531]
Food-derived phytochemicals, many known for their health beneficial effects, often exist in conjugated forms containing sugar moieties such as glucose or rhamnose in foods. The uptake of these compounds requires colonic bacterial cleavage of sugar moieties. However, most studies involved in screening extracts for biological activities do not take this process into account. This study seeks to determine the utility of commercially available hesperidinase to mimic colonic digestion and to test the effects of this treatment on the biological properties of extracts. Using hesperidinase resulted in efficient hydrolysis of Engelhardia roxburghiana Wall. extract containing rhamnose conjugates. Enzymatic digestion enhanced the extract's cellular antioxidant ability by 2-fold in HepG2/C3A and the anti-inflammatory effect on lipopolysaccharide-induced interleukin (IL)-1β and IL-6 expression in mouse macrophage J774A.1 and human monocyte THP-1 cells. Enzymatic digestion also efficiently processed extracts with mixed rhamnose and glucose conjugates and altered their biological activities. Results of the present study supported the importance of considering enzymatic digestion during the biological activity studies of botanicals.
Secondary metabolites from the stems of Engelhardia roxburghiana and their antitubercular activities.[Pubmed: 22818359]
Bioassay-guided fractionation of stems of Engelhardia roxburghiana led to isolation of: four diarylheptanoids, engelheptanoxides A-D (1-4); two cyclic diarylheptanoids, engelhardiols A (5) and B (6); one naphthoquinone dimer, engelharquinonol (7); and one 1-tetralone, (4S)-4,6-dihydroxy-1-tetralone (8), along with 24 known compounds (9-32). The structures of 1-8 were by spectroscopic analysis. Compounds 5, 6, 13, 22, and 23 showed antitubercular activity against Mycobacterium tuberculosis H(37)Rv with MIC values of 72.7, 62.1, 9.1, 15.3, and 70.1μM, respectively.
Isolation and characterization of two flavonoids, engeletin and astilbin, from the leaves of Engelhardia roxburghiana and their potential anti-inflammatory properties.[Pubmed: 21476602]
Engeletin, a flavonoid compound, was isolated from the leaves of Engelhardia roxburghiana for the first time, along with astilbin, another flavonoid. The chemical structures of engeletin and astilbin were confirmed by (1)H and (13)C nuclear magnetic resonance (NMR) and mass spectrometry (MS) spectra, and their anti-inflammatory activities were studied in lipopolysaccharide (LPS)-stimulated mouse J774A.1 macrophage cells. LPS induced the inflammatory state in macrophage cells and increased mRNA expressions of pro-inflammatory cytokines. Engeletin and astilbin exhibited remarkable inhibitory effects on interleukin (IL)-1β and IL-6 mRNA expression. Significant inhibition of LPS-mediated mRNA expressions were also seen in LPS binding toll-like receptor (TLR)-4, pro-inflammatory cytokine tumor necrosis factor (TNF)-α, IL-10, chemoattractant monocyte chemotactic protein (MCP)-1, and cyclooxygenase (COX)-2 genes. The reduced expression of these cytokines may alleviate immune response and reduce inflammatory activation, indicating that engeletin and astilbin may serve as potential anti-inflammatory agents.
Secondary metabolites from the roots of Engelhardia roxburghiana and their antitubercular activities.[Pubmed: 17418287]
Engelharquinone (1), engelharquinone epoxide (2), engelharolide (3), and engelhardic acid (4), were isolated as naturally occurring products from a plant source, Engelhardia roxburghiana together with 20 previously known compounds, four of which were hitherto not known as plant constituents. Their structures were identified by means of spectroscopic analysis. A biological evaluation showed that three of the previously isolated antitubercular constituents [(-)-4-hydroxy-1-tetralone, 3-methoxyjuglone and engelhardione] and engelharquinone (1) exhibited moderate antitubercular activity against Mycobacterium tuberculosis 90-221387.
Antitubercular constituents from the roots of Engelhardia roxburghiana.[Pubmed: 15729627]
Three new compounds, engelhardione, (-)-5-hydroxy-4-methoxy-1-tetralone, and 3-methoxycarbonyl-1,5-dihydroxyanthraquinone, together with twelve known compounds have been isolated from the roots of Engelhardia roxburghiana. The structures of these new compounds were determined through spectral analyses. Engelhardione, 3-methoxyjuglone, and (-)-4-hydroxy-1-tetralone showed antitubercular activities with MIC values=3.125, 3.125, and 6.25 microg/mL against Mycobacterium tuberculosis 90-221,387, respectively, and with MIC values=0.2, 0.2, and 4.0 microg/mL against M. tuberculosis H37Rv, individually.


