Euphorbia fischeriana
Euphorbia fischeriana
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
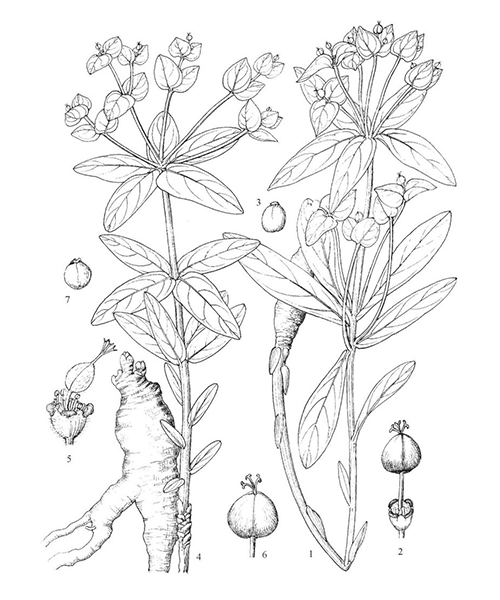
Natural products/compounds from Euphorbia fischeriana
- Cat.No. Product Name CAS Number COA
-
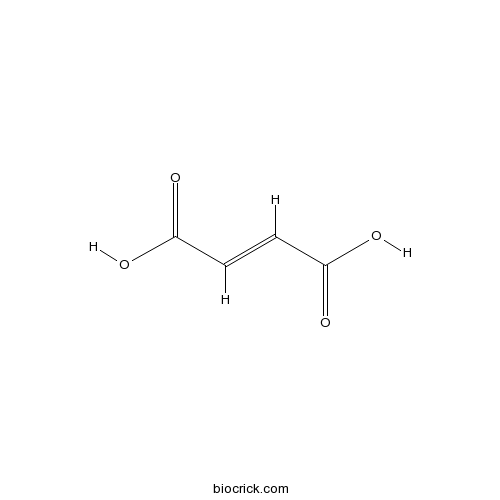
BCN5989
Fumaric acid110-17-8
Instructions
-
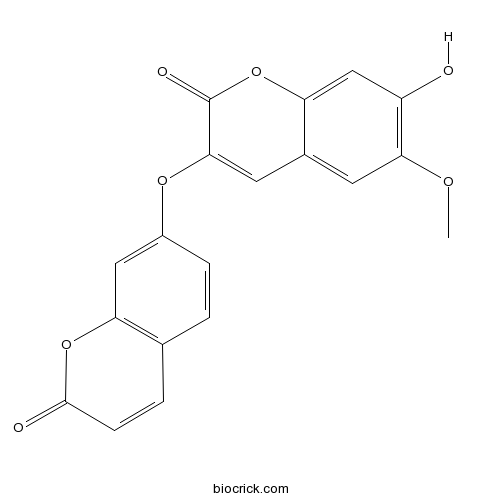
BCN2473
Daphnoretin2034-69-7
Instructions
-
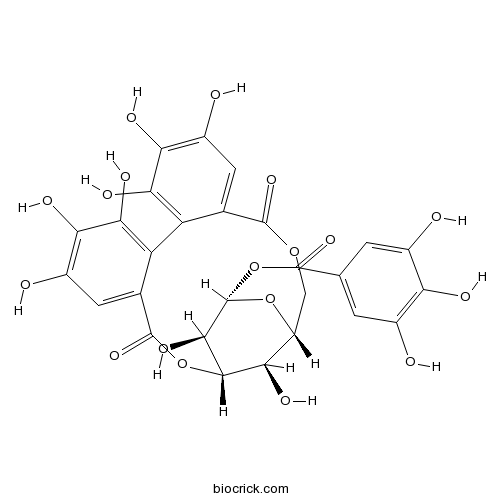
BCN2322
Corilagin23094-69-1
Instructions
-
BCN2333
Ingenol30220-46-3
Instructions
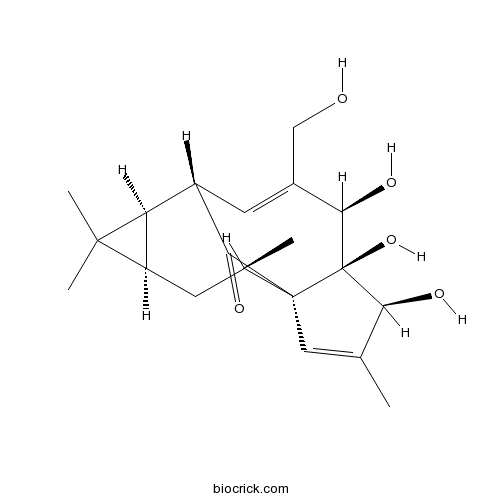
-
BCN2391
Jolkinolide B37905-08-1
Instructions
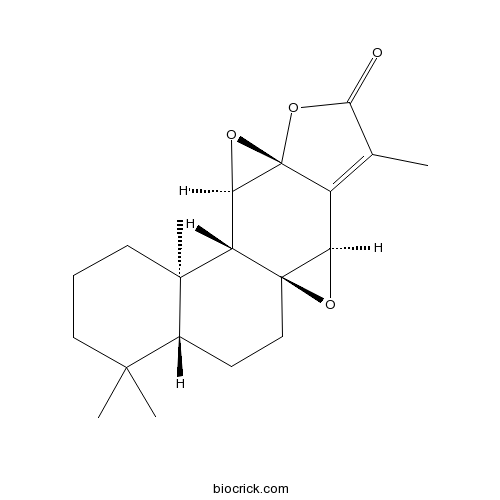
-
BCN3181
Campesterol474-62-4
Instructions
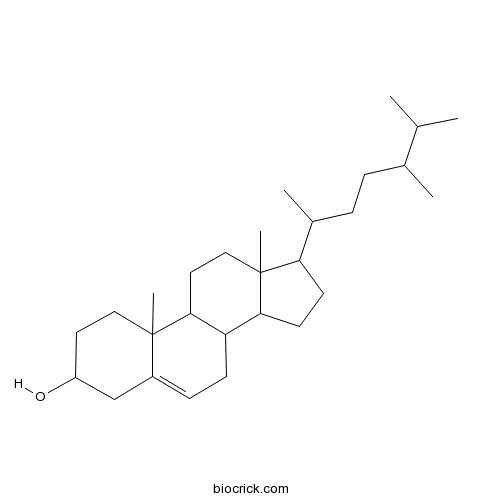
-
BCN5701
Scopolin531-44-2
Instructions
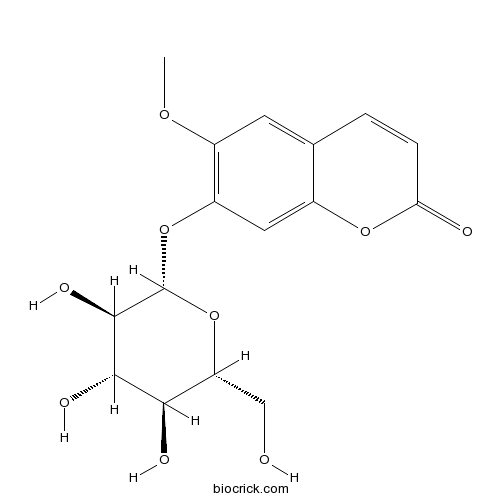
-
BCN5725
Lupeol545-47-1
Instructions

-
BCN1015
Beta-Sitosterol83-46-5
Instructions
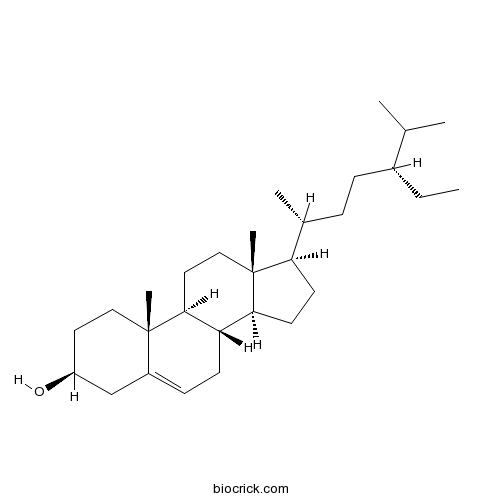
-
BCN4376
Stigmasterol83-48-7
Instructions
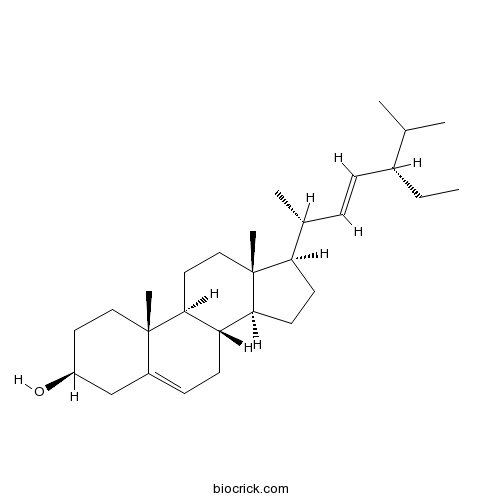
-
BCN4373
Ethyl gallate831-61-8
Instructions

-
BCN4470
Scopoletin92-61-5
Instructions

Anti-Cancer Activities of Diterpenoids Derived from Euphorbia fischeriana Steud.[Pubmed: 29439483]
None
Chemical profiling of Euphorbia fischeriana Steud. by UHPLC-Q/TOF-MS.[Pubmed: 29324281]
None
Diterpenoids from the roots of Euphorbia fischeriana and their inhibitory effects on α-glucosidase.[Pubmed: 28944698]
Chemical investigation has been performed on the roots of Euphorbia fischeriana, a traditional Chinese medicine. Three diterpenoids were obtained using various chromatographic techniques, and their structures were determined by spectroscopic data including HRESIMS, 1D NMR, 2D NMR, ECD, and calculated ECD, which gave two new diterpenoids, daphnane type (1) and ent-pimarene type (3). Additionally, the isolated compounds (1-3) displayed moderate inhibitory effects against α-glucosidase in an in vitro bioassay.
Development of a Matrix Solid-Phase Dispersion Extraction Combined with UPLC/Q-TOF-MS for Determination of Phenolics and Terpenoids from the Euphorbia fischeriana.[Pubmed: 28892000]
None
ent-Abietane and Tigliane Diterpenoids from the Roots of Euphorbia fischeriana and Their Inhibitory Effects against Mycobacterium smegmatis.[Pubmed: 28383891]
An investigation on the bioactive chemical constituents of the roots of Euphorbia fischeriana has been conducted, with 21 diterpenoids obtained using various chromatographic techniques. On the basis of spectroscopic data analysis, the new compounds were elucidated as four ent-abietane-type diterpenoids (1-4) and four tigliane-type diterpenoids (13-16). Also obtained were eight known ent-abietane (5-12) and five known tigliane (17-21) diterpenoids. The potential antituberculosis effects of these diterpenoids were evaluated using a Mycobacterium smegmatis model. The most potent compound according to the in vitro bioassay used was 17-hydroxyjolkinolide B (12) (MIC 1.5 μg/mL).
Development of matrix solid-phase dispersion coupled with high-performance liquid chromatography for determination of jolkinolide A and jolkinolide B in Euphorbia fischeriana Steud.[Pubmed: 28338342]
None
Jolkinolide A and Jolkinolide B Inhibit Proliferation of A549 Cells and Activity of Human Umbilical Vein Endothelial Cells.[Pubmed: 28087861]
BACKGROUND Jolkinolide A (JA) and Jolkinolide B (JB) are diterpenoids extracted from the roots of Euphorbia fischeriana Steud and have been shown to have anti-tumor activity. However, their effects on the ability of tumor cells to invade blood vessels and metastasize remain largely unknown. Investigations into the effects of JA and JB on the angiogenesis of tumor tissues may facilitate the identification of new natural drugs with anti-tumor growth and metastasis activities. MATERIAL AND METHODS We used different concentrations of JA and JB (20 μg/ml, 40 μg/ml, 60 μg/ml, 80 μg/ml, and 100 μg/ml) to stimulate A549 cells and then studied the effects on the growth and metastasis of lung cancers. In addition, we used conditional media from A549 cells (A549-CM) stimulated by either JA or JB in different concentrations to culture human umbilical vein endothelial cells (HUVECs). RESULTS We found that both JA and JB significantly inhibited the Akt-STAT3-mTOR signaling pathway and reduced the expression of VEGF in A549 cells, but JB exhibited more significant inhibitory effects than JA. The JB-stimulated A549 cell conditional media had a greater inhibitory effect on the proliferation and migration of HUVECs than did the conditional media of JA-stimulated A549 cells. This effect gradually increased with increasing concentrations of either type of Jolkinolide. CONCLUSIONS Our results suggest that JA and JB inhibited VEGF expression in A549 cells through the inhibition of the Akt-STAT3-mTOR signaling pathway, and directly inhibited the proliferation and migration of HUVECs. These findings are of great significance for the development of new plant-derived chemotherapy agents for the treatment of cancer.


