Hypericum sampsonii
Hypericum sampsonii
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Hypericum sampsonii
- Cat.No. Product Name CAS Number COA
-
BCN5948
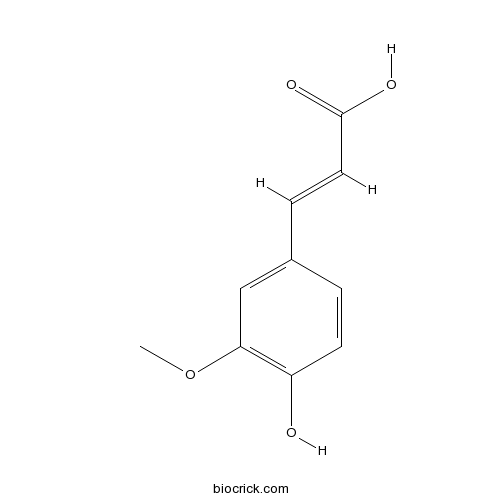
Ferulic acid1135-24-6
Instructions
-
BCN5653
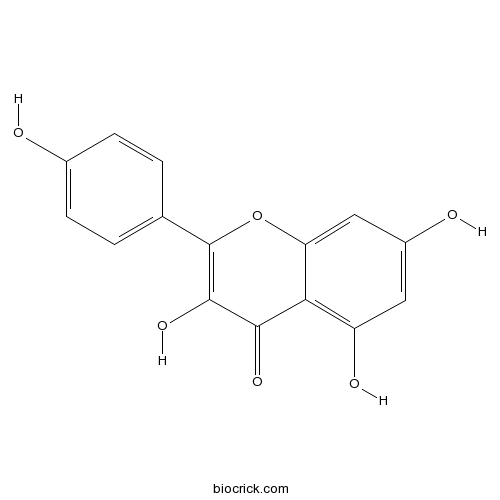
Kaempferol520-18-3
Instructions
-
BCN5977
Hypericin548-04-9
Instructions

-
BCN2973
1-Octacosanol557-61-9
Instructions

-
BCN4546
4-Hydroxybenzoic acid99-96-7
Instructions

Chiral separation and absolute configurations of two pairs of racemic polyprenylated benzophenones from Hypericum sampsonii.[Pubmed: 27818319]
(±) Sampsonins A-B (1-2), two pairs of racemic polyprenylated benzophenones, were isolated from the aerial parts of Hypericum sampsonii and successfully separated by chiral HPLC column. Their structures were elucidated by spectroscopic analyses, X-ray diffraction analysis, and quantum chemical calculation of ECD method. Besides, the plausible biogenetic pathways of 1-2 were proposed, and all of them were evaluated for RXRα transcriptional-inhibitory activities and cytotoxicity against HeLa cells.
Hyperisampsins H-M, Cytotoxic Polycyclic Polyprenylated Acylphloroglucinols from Hypericum sampsonii.[Pubmed: 26440674]
Six new polycyclic polyprenylated acylphloroglucinols (PPAPs), named hyperisampsins H-M (1-6), were isolated from the aerial parts of Hypericum sampsonii, together with five known analogs (7-11). The structures of 1-6 were established by extensive spectroscopic analyses, including HRESIMS and NMR. In addition, the absolute configurations of these new compounds were determined by electronic circular dichroism (ECD) calculations. Compounds 1 and 2 represent the first examples of PPAPs possessing a unique γ-lactone ring at C-23, while 3-6 differed from normal PPAPs with an unprecedented 1,2-dioxane ring. Compounds 1-7 were evaluated for their cytotoxic activities against a panel of human cancer cell lines in vitro, of which 3, 4, and 6 exhibited significant cytotoxic activities with IC50 values ranging from 0.56 to 3.00 μM. Moreover, compound 3 induces leukemia cell apoptotic death, evidenced by activation of caspase-3, degradation of PARP, up-regulation of Bax, and down-regulation of Bcl-2 and Bcl-xl.
Dioxasampsones A and B, two polycyclic polyprenylated acylphloroglucinols with unusual epoxy-ring-fused skeleton from Hypericum sampsonii.[Pubmed: 25470320]
Dioxasampsones A and B (1 and 2), two new polycyclic polyprenylated acylphloroglucinols with an unusual epoxy-ring-fused skeleton by new ways of cyclization, along with a new nor-PPAPs hypersampson R (3) with the loss of C-31-33 in isopentenyl, were isolated from the aerial parts of Hypericum sampsonii. 1 possessed an unexpected hexacyclic skeleton with a rare 2,7-dioxabicyclo[2.2.1]heptane moiety, and 2 featured a unique tetrahydrofuro[3,4-b]furan-fused tricycle[4.3.1.1(5,7)]undecane skeleton. The gross structures of the new compounds were determined by extensive NMR spectroscopic methods. Their absolute configurations were deduced by single-crystal X-ray diffraction and ECD calculations.
Novel polyprenylated phloroglucinols from Hypericum sampsonii.[Pubmed: 25460308]
Hypericum sampsonii Hance (Clusiaceae) is a folk medicine used in Taiwan to treat blood stasis, relieve swelling, and as an anti-hepatitis drug. Two new polyprenylated phloroglucinol derivatives, hypersampsone R (1) and hypersampsone S (2), and a known prenylated benzophenone, hyperibone K (3) were isolated from the aerial parts of H. sampsonii. Their structures were determined by extensive 1D and 2D NMR, and MS spectral analyses.
Bioactive acylphloroglucinols with adamantyl skeleton from Hypericum sampsonii.[Pubmed: 25453445]
Hyperisampsins A-D (1-4), with tetracyclo[6.3.1.1(3,10).0(3,7)]tridecane skeletons and seven biogenetically related congeners (5-11), were isolated from Hypericum sampsonii. Their structures were elucidated by comprehensive spectroscopic techniques. The absolute configuration of 1 was established by ECD calculations, and those of 5 and 9 were confirmed by single X-ray crystallographic analyses. Hyperisampsins A and D showed potent anti-HIV activities with EC50 of 2.97 and 0.97 μM and selectivity index of 4.80 and 7.70, respectively.
Norsampsones A-D, four new decarbonyl polycyclic polyprenylated acylphloroglucinols from Hypericum sampsonii.[Pubmed: 24932990]
Norsampsones A-D (1-4), four new decarbonyl polycyclic polyprenylated acylphloroglucinols, together with a new biogenetically related compound hypersampsone M (5), were isolated from the aerial parts of Hypericum sampsonii. Norsampsones A-D featured an unprecedented carbon skeleton with the loss of C-2 carbonyl in the phloroglucinol ring. All structures were determined by extensive NMR spectroscopic methods, ECD calculation, and single-crystal X-ray diffraction.
Differential expression of benzophenone synthase and chalcone synthase in Hypericum sampsonii.[Pubmed: 23413566]
cDNAs encoding Hypericum sampsonii benzophenone synthase (HsBPS) and chalcone synthase (HsCHS) were isolated and functionally characterized. Differential expressions of HsBPS and HsCHS were monitored using quantitative polymerase chain reaction (PCR). In the vegetative stage, HsBPS was highly expressed in the roots; its transcript level was approx. 100 times higher than that of HsCHS. Relatively high transcript amounts of HsBPS were also detected in older leaves, whereas the youngest leaves contained higher transcript amounts of HsCHS. In the reproductive stage, maximum HsCHS expression was detected in flowers, the transcript level being approx. 5 times higher than that of HsBPS. The inversed situation with a 10-fold difference in the expression levels was observed with fruits. High transcript amounts for both proteins were found in roots.
Prenylated phloroglucinol derivatives from Hypericum sampsonii.[Pubmed: 22981504]
Six new acylphloroglucinol derivatives, sampsonols A-F (1-6), were isolated from the petroleum ether extract of the aerial parts of Hypericum sampsonii. The structures and relative configurations of sampsonols A-F were elucidated by extensive spectroscopic analyses. All these compounds were tested for their in vitro cytotoxic and anti-inflammatory activities. Sampsonols A and B (1 and 2) showed significant cytotoxicity against four human tumor cell lines with IC(50) values in the range of 13-28μM, whereas sampsonols C and F (3 and 6) showed potent inhibitory activities against LPS-induced NO production in RAW 264.7 macrophages with IC(50) values of 27.3 and 29.3μM, respectively.
Two new xanthones from Hypericum sampsonii and biological activity of the isolated compounds.[Pubmed: 20839213]
Phytochemical investigation of the CH(2) Cl(2) extract of the aerial part of Hypericum sampsonii yielded two new prenylated xanthones, hypericumxanthone A and B, together with three known xanthones. Their structures were elucidated by analysis of physical and spectral (UV, IR, mass and NMR) data and comparison of spectroscopic data with those reported previously. All these compounds were evaluated for in vitro antibacterial activity against methicillin-resistant Staphylococcus aureus (MRSA). Two new compounds were also tested for their cytotoxicity against human breast (MCF-7), hepatoma (HepG2), colon (HT-29) and lung (A549) tumour cell lines. Two new compounds showed moderate antibacterial activities at minimum inhibitory concentrations (MIC) of 16 and 32 µg/mL, respectively, whereas the positive standard antibacterial drug, vancomycin, showed an MIC of 8 µg/mL. The other compounds were inactive against MRSA. In addition, hypericumxanthone B showed weak inhibitory activities against four human tumour cell lines.


