Inula japonica
Inula japonica
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Inula japonica
- Cat.No. Product Name CAS Number COA
-
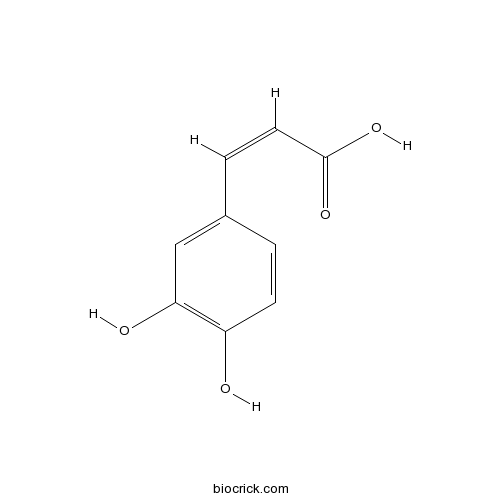
BCN5979
Caffeic acid331-39-5
Instructions
-
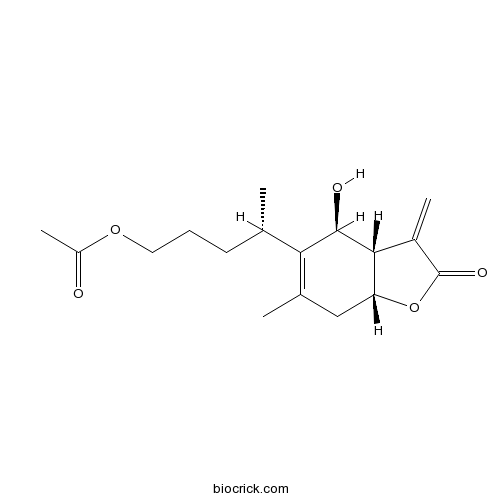
BCN7715
1-O-Acetylbritannilactone33627-41-7
Instructions
-
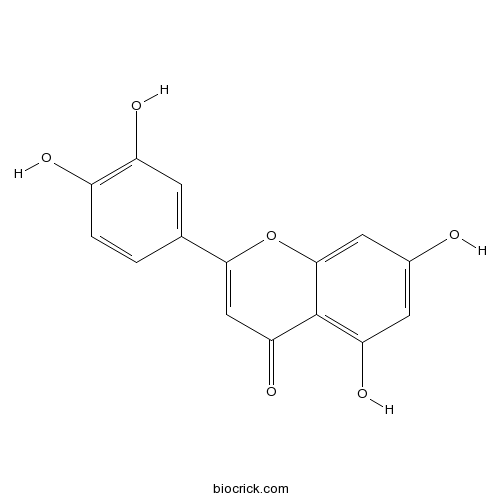
BCN5600
Luteolin491-70-3
Instructions
-
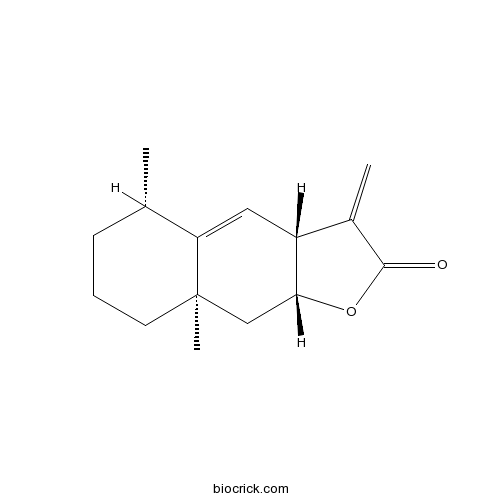
BCN1033
Alantolactone546-43-0
Instructions
Dimeric- and trimeric sesquiterpenes from the flower of Inula japonica.[Pubmed: 30096515]
None
NO inhibitors function as potential anti-neuroinflammatory agents for AD from the flowers of Inula japonica.[Pubmed: 29421695]
The extensive pathology studies revealed that Alzheimer's disease (AD) is closely related to neuroinflammation and anti-neuroinflammatory agents may be potentially useful for the treatment of AD. Inula japonica is a member of the Asteraceae plant family and its flowers have been used as a healthy tea and a traditional Chinese medicine. Our continuous search for new nitric oxide (NO) inhibitory substances as anti-neuroinflammatory agents for AD resulted in the isolation of two new sesquiterpenes and ten known terpenes from the flowers of I. japonica. Their structures were established on the basis of extensive analysis of NMR and MS spectroscopic data, as well as calculated and experimental electronic circular dichroism (ECD) spectra. Among these isolates, compound 1 is a new sesquiterpene with a rare tricyclic fused skeleton, and 2 processes a 1,10-seco-eudesmane skeleton. The anti-neuroinflammatory effects were examined by inhibiting NO release in LPS-induced murine microglial BV-2 cells. The possible mechanism of NO inhibition was also investigated using molecular docking, which revealed the interactions of bioactive compounds with the iNOS protein. The present study disclosed that the flowers of I. japonica as a healthy tea are potentially useful for AD and related neuroinflammatory diseases.
Structural characters and protecting β-cells of a polysaccharide from flowers of Inula japonica.[Pubmed: 28286076]
None
Bioactive sesquiterpenoids from the flowers of Inula japonica.[Pubmed: 27452450]
Phytochemical investigation of the flowers of Inula japonica led to isolation of nine sesquiterpenoids, inujaponins A-I, as well as eighteen known ones. These sesquiterpenoids belong to six skeletal-types, including eudesmane, 1,10-seco-eudesmane, germacrane, guaiane, 4,5-seco-guaiane, and pseudoguaiane sesquiterpenoids. Their structures were established by extensive spectroscopic analysis. The absolute configurations of inujaponin A, eupatolide, and deacetylovatifolin were determined by Cu-Kα X-ray crystallographic analysis. Most of the isolated compounds exhibited potent cytotoxicity against HL-60, SMMC-7721, A-549, MCF-7, and SW-480 cancer cell lines, with IC50 values ranging from 1.57 to 22.58 μM. Some selected compounds also possessed significant inhibitory activity against LPS-induced NO production in RAW264.7 macrophages with IC50 values ranging from 1.42 to 8.99 μM.
Sesquiterpenes from Inula japonica with Inhibitory Effects on Nitric Oxide Production in Murine Macrophage RAW 264.7 Cells.[Pubmed: 27276091]
Eight new sesquiterpenes (1-8), along with seven known sesquiterpenes (9-15), were isolated from a methanol extract of the flowers of Inula japonica. Their structures were elucidated by a combination of 1D and 2D NMR spectroscopic and HRESIMS data. All of isolates were evaluated for their inhibitory effects on nitric oxide production in LPS-induced RAW 264.7 cells, with their IC50 values ranging from 1.9 to 15.4 μM.
KOTMIN13, a Korean herbal medicine alleviates allergic inflammation in vivo and in vitro.[Pubmed: 27267050]
The ethanol extract of KOTMIN13, composed of Inula japonica Flowers, Trichosanthes kirilowii Semen, Peucedanum praeruptorum Radix, and Allium macrostemon Bulbs, was investigated for its anti-asthmatic and anti-allergic activities.
DNA Topoisomerase Inhibitory Activity of Constituents from the Flowers of Inula japonica.[Pubmed: 26936053]
Fourteen compounds were isolated from the flowers of Inula japonica THUNB. (Asteraceae), including two new compounds, (1S,2S,4S,5S,8S,10R)-2-acetoxy-4,3-dihydroxy-pseudoguai-7(11)-en-12,8-olide (1) and (1S,2S,4S,5S,8S,10R)-2,4,13-trihydroxy-pseudoguai-7(11)-en-12,8-olide (2), and twelve known compounds, budlein B (3), 6β-hydroxytomentosin (4), 6-deacetoxybritanin (5), 4-epipulchellin (6), britanin (7), tomentosin (8), (+)-dihydroquercetin (9), (-)-syringaresinol (10), quercetagetin 3,4'-dimethyl ether (11), luteolin (12), britanin G (13) and inuchinenolide C (14). Structures of 1 and 2 were determined based on one and two dimensional (1D)- and (2D)-NMR data and Mosher's esterification method. Compounds 9 and 12 showed inhibitory activities toward DNA topoisomerase I with IC50 values of 55.7 and 37.0 µM, respectively, compared to camptothecin (CPT) with an IC50 of 24.5 µM. Compounds 7-9 and 11-14 exhibited more potent inhibitory activity against topoisomerases II with IC50 values of 6.9, 3.8, 3.0, 6.9, 10.0, 14.7 and 13.8 µM, respectively, than that of etoposide (VP-16) with an IC50 of 26.9 µM. Compounds 4-7 and 10-14 exhibited weak cytotoxicities to the selected cancer cell lines.


