Kadsura coccinea
Kadsura coccinea
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Kadsura coccinea
- Cat.No. Product Name CAS Number COA
-
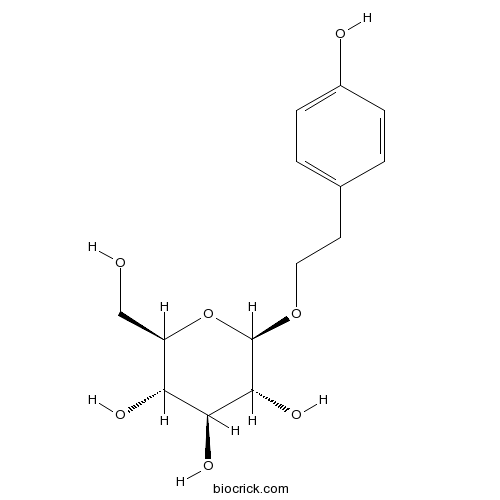
BCN5966
Salidroside10338-51-9
Instructions
-
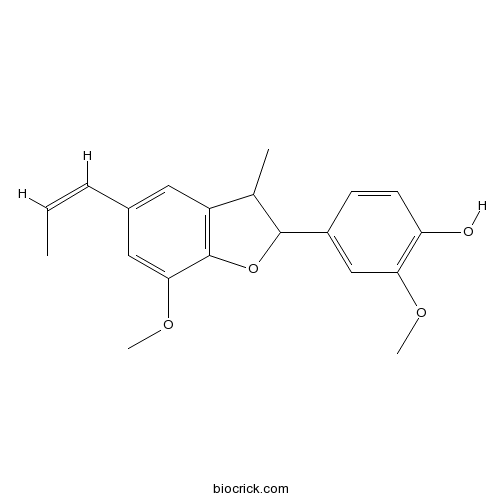
BCN1240
Dehydrodiisoeugenol2680-81-1
Instructions
-
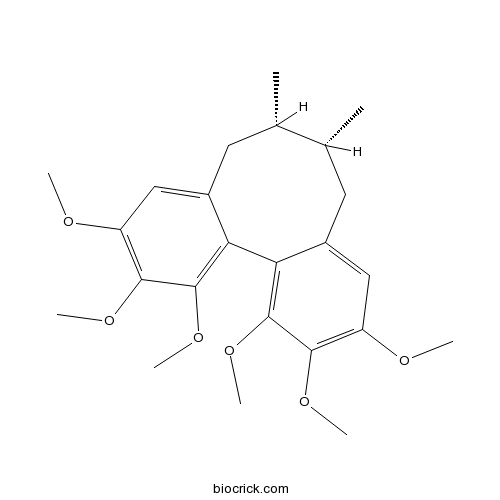
BCN1021
Schizandrin A61281-38-7
Instructions
-
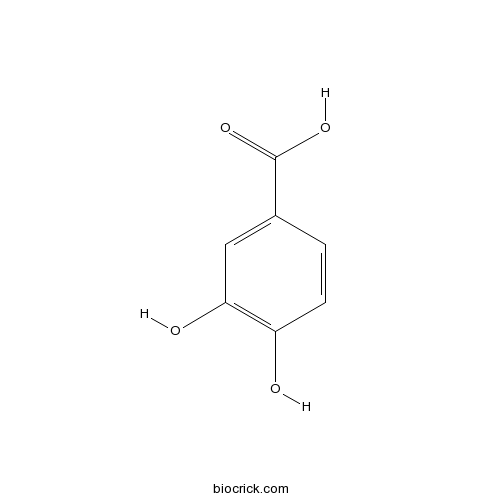
BCN4537
3,4-Dihydroxybenzoic acid99-50-3
Instructions
Rearranged 6/6/5/6-Fused Triterpenoid Acids from the Stems of Kadsura coccinea.[Pubmed: 27704807]
None
Structural Characterization of Kadcoccinin A: A Sesquiterpenoid with a Tricyclo[4.4.0.0(3,10)]decane Scaffold from Kadsura coccinea.[Pubmed: 27091303]
Kadcoccinin A (1), a cage-like sesquiterpenoid possessing a tricyclo[4.4.0.0(3,10)]decane scaffold, and the biosynthetically related kadcoccinin B (2) were isolated from the stems of Kadsura coccinea. Their structures and absolute configurations were determined from extensive spectroscopic analysis and quantum chemical calculations. Additionally, their cytotoxic and antifungal effects were initially evaluated, and a plausible biosynthetic pathway was proposed.
A high-performance liquid chromatography with circular dichroism detector for determination of stereochemistry of 6, 9-oxygen bridge dibenzocyclooctadiene lignans from kadsura coccinea.[Pubmed: 26481381]
The stereochemistry of two 6, 9-oxygen bridge dibenzocyclooctadiene lignans from Kadsura coccinea, are difficult to separate and very unstable. The present study was designed to develop a high-performance liquid chromatography using circular dichroism detection for the analysis of the stereochemistry. A new 6, 9-oxygen bridge dibenzocyclooctadiene lignans named Kadsulignan Q was firstly found with an S-biphenyl configuration. The other compound was identified as Kadsulignan L with an R- biphenyl configuration. In order to obtain kinetic data on their reversible interconversion, the stability was measured at different deuterated solvents such as deuterated methanol, deuterated chloroform and deuterated dimethylsulfoxide. The lignans were more unstable and converted more easily in deuterated methanol than in deuterated chloroform and deuterated dimethylsulfoxide.
Kadcoccinones A-F, New Biogenetically Related Lanostane-Type Triterpenoids with Diverse Skeletons from Kadsura coccinea.[Pubmed: 26348535]
Six new lanostane-related triterpenoids, kadcoccinones A-F (1-6), were isolated from Kadsura coccinea. Compound 3 possesses a novel 6/6/9-fused carbocyclic core containing a rare oxabicyclo[4.3.1]decane system. Compounds 4 and 5 are isomers representing the first example of the 18(13 → 12)-abeo-26-norlanostane triterpenoid. The absolute configurations of 1 and 4-6 were defined by X-ray diffraction and experimental ECD spectra, and that of 3 was elucidated by quantum chemical calculations. The plausible biogenetic pathway of 1-6 is postulated.
Kadcoccinic Acids A-J, Triterpene Acids from Kadsura coccinea.[Pubmed: 26214125]
Eleven triterpene acids including 10 new compounds (kadcoccinic acids A-J, 1-10) were isolated from the stems of Kadsura coccinea. Except for 10, these compounds feature a rearranged lanostane skeleton with a 6/6/5/6 tetracyclic ring system, and compounds 1 and 2 are the first examples of 2,3-seco-6/6/5/6-fused tetracyclic triterpenoids. Their structures were established primarily by spectroscopic and spectrometric methods. Additionally, the absolute configuration of 3 was determined by single-crystal X-ray diffraction. Several of the compounds isolated were tested for their anti-HIV-1 and cytotoxic activities.
A lignin glycoside and a nortriterpenoid from Kadsura coccinea.[Pubmed: 25443372]
To study the chemical constituents of the roots and stem bark of Kadsura coccinea.
Kadcotriones A-C: tricyclic triterpenoids from Kadsura coccinea.[Pubmed: 24299567]
Three new triterpenoids, kadcotriones A-C (1-3), together with the biogenetically related lanostane-type triterpenoid (4), were isolated from Kadsura coccinea. Compound 1 features a 12,14β-dimethyl 6/6/6-fused tricyclic skeleton, while 2 and 3 are characterized by a 6/6/5-ring system. Their structures were determined by NMR and electronic circular dichroism spectroscopic methods. Compounds 2 and 4 exhibited anti-HIV-1 activities with EC50 values of 30.29 and 54.81 μM, respectively.
Cytotoxic dibenzocyclooctadiene lignans from Kadsura coccinea.[Pubmed: 23784205]
Three new dibenzocyclooctadiene lignans, kadusurain A-C (1-3), together with two known compounds kadsuphilin A (4) and B (5), were isolated from an EtOAc fraction of the 80 % acetone extract of Kadsura coccinea (Lem.) A. C. Smith. Their structures were established by 1D and 2D NMR techniques, and mass spectroscopy. Anti-proliferative effect of isolated compounds was evaluated against four human tumor cell lines (A549, HCT116, HL-60, and HepG2), and it was found that compound 1 exhibited significant antiproliferative effects with IC50 values ranging from 1.05 to 12.56 μg/ml.
Depigmentation effect of kadsuralignan F on melan-a murine melanocytes and human skin equivalents.[Pubmed: 23322017]
The development of melanogenic inhibitors is important for the prevention of hyperpigmentation, and, recently, consideration has been given to natural materials or traditionally used ingredients such as Chinese medicine. The aim of this study is the evaluation of a new anti-melanogenic candidate, kadsuralignan F, from the natural plant Kadsura coccinea, as well as the determination of mechanisms of melanogenesis inhibition at a molecular level. Kadsuralignan F significantly reduced melanin synthesis in a dose-dependent manner in a murine melanocyte cell line and human skin equivalents. There was no direct inhibition on mushroom tyrosinase or cell-extract tyrosinase activity, and mRNA expression of tyrosinase and other melanogenic genes such as tyrosinase-related protein-1 (trp-1) or trp-2 were not affected by kadsuralignan F. Interestingly, the protein level of tyrosinase was dramatically downregulated with kadsuralignan F treatment. We found that a decrease of tyrosinase protein by kadsuralignan F was fully recovered by MG132, a proteasome inhibitor, but not by chloroquine, a lysosome inhibitor. In this study, we found that kadsuralignan F, a lignan from an extract of Kadsura coccinea, has an inhibitory activity on melanin synthesis through tyrosinase degradation. These findings suggest that kadsuralignan F can be used as an active ingredient for hyperpigmentation treatment.
Kadcoccitones A and B, two new 6/6/5/5-fused tetracyclic triterpenoids from Kadsura coccinea.[Pubmed: 23230829]
A pair of new triterpenoid epimers, kadcoccitones A (1) and B (2), together with a new biogenetically related compound kadcoccitone C (3), were isolated from Kadsura coccinea. The epimers featured an unprecedented carbon skeleton with a 6/6/5/5-fused tetracyclic ring system unit and a C(9) side chain. Their structures were determined by spectroscopic data, ECD calculation, and single-crystal X-ray diffraction. Compounds 1 and 3 showed anti-HIV-1 activity with an EC(50) value of 47.91 and 32.66 μg/mL, respectively.


