Peucedanum praeruptorum
Peucedanum praeruptorum
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
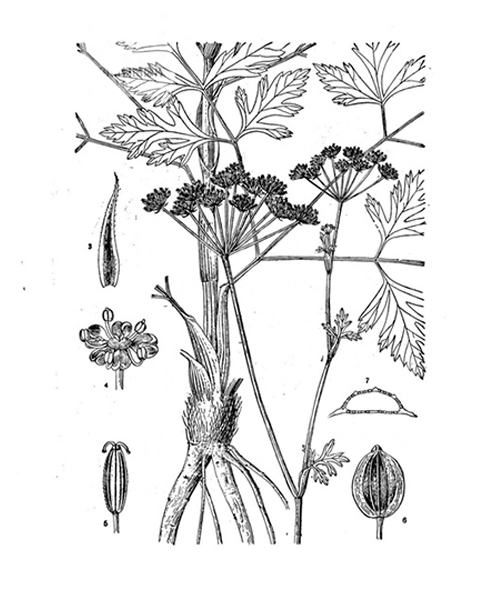
Natural products/compounds from Peucedanum praeruptorum
- Cat.No. Product Name CAS Number COA
-
BCN6059
Syringin118-34-3
Instructions

-
BCN3470
(+)-Pteryxin13161-75-6
Instructions

-
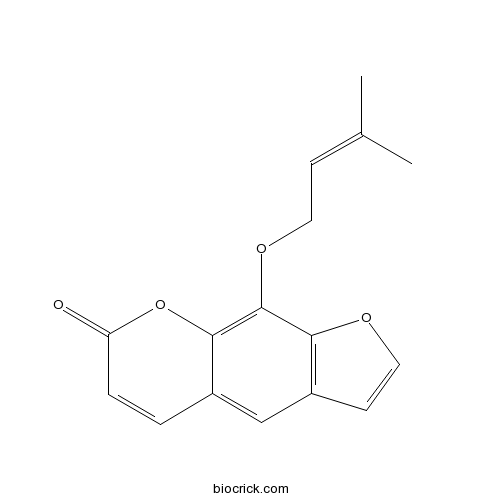
BCN5574
Imperatorin482-44-0
Instructions
-
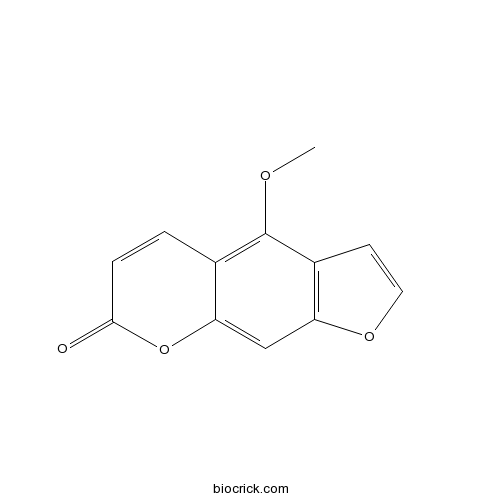
BCN5582
Bergapten484-20-8
Instructions
-
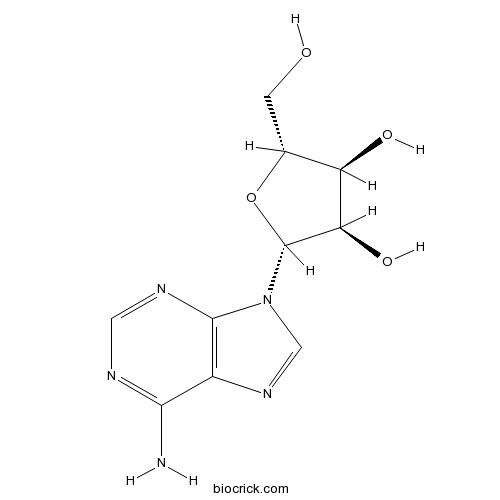
BCN5796
Adenosine58-61-7
Instructions
-
BCN2205
D-Mannitol69-65-8
Instructions

-
BCN4989
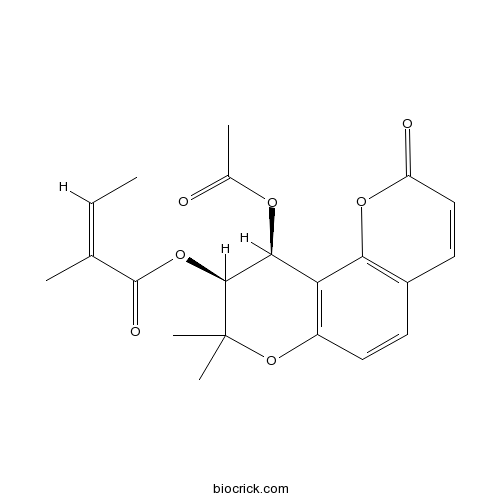
(+)-Praeruptorin A73069-27-9
Instructions
-
BCN4990
Praeruptorin D73069-28-0
Instructions

-
BCN2591
Praeruptorin E78478-28-1
Instructions

[Study of extracting Peucedanum praeruptorum planted area in Ningguo of Anhui province based on multi-source and multi-phase image].[Pubmed: 29318836]
The herbs used as the material for traditional Chinese medicine are always planted in the mountainous area where the natural environment is suitable. As the mountain terrain is complex and the distribution of planting plots is scattered, the traditional survey method is difficult to obtain accurate planting area. It is of great significance to provide decision support for the conservation and utilization of traditional Chinese medicine resources by studying the method of extraction of Chinese herbal medicine planting area based on remote sensing and realizing the dynamic monitoring and reserve estimation of Chinese herbal medicines. In this paper, taking the Peucedanum praeruptorum planted area in Ningguo prefecture of Anhui province as an example, the multispectral remote sensing images that include Landsat-8 with a 30 m resolution and China-made GF-1 with a 16 m resolution were used as data source. Since the spectral characteristics of P. praeruptorum in the two periods are different from those of other crops, the changes of the images at two stages in the same year could be used to extract the P. praeruptorum planted area intercropped in cultivated land. Then the texture and spectral characteristics of young pecan trees were used to extract the P. praeruptorum planted area intercropped in woodland. The results showed that the extracted area of planted P. praeruptorum with the original imagery of 30 m spatial resolution and 16 m spatial resolution was 25 635.43,24 585.43 mu, respectively.
Identification and functional characterization of a p-coumaroyl CoA 2'-hydroxylase involved in the biosynthesis of coumarin skeleton from Peucedanum praeruptorum Dunn.[Pubmed: 28822035]
A p-coumaroyl CoA 2'-hydroxylase responsible for the formation of coumarin lactone ring was identified from Peucedanum praeruptorum Dunn and functionally characterized in vitro. Coumarins are important plant secondary metabolites with a variety of biological activities. Ortho-hydroxylation of cinnamates leads to the formation of coumarin lactone ring and is generally thought to be a key step in coumarin biosynthesis. However, ortho-hydroxylases, especially p-coumaroyl CoA 2'-hydroxylase (C2'H) responsible for the biosynthesis of the most common coumarin skeleton, have received insufficient attention. Here, a putative ortho-hydroxylase PpC2'H was isolated from P. praeruptorum Dunn, a traditional Chinese medicinal herb rich in coumarins. Expression profile indicated that PpC2'H exhibited the highest transcript level in roots and could be up-regulated by MeJA elicitation. Subcellular localization of PpC2'H was demonstrated to be cytosol in planta. In order to functionally characterize PpC2'H, the purified recombinant protein was incubated with various potential substrates. HPLC-ESI-MS analysis indicated that PpC2'H catalyzed the conversion of p-coumaroyl CoA into hydroxylated intermediate, which then underwent spontaneous lactonization to generate umbelliferone. Our data also showed that light would promote the spontaneous process. In addition, based on homology modeling and site-directed mutagenesis, amino acid residues Phe-130, Lys-141, Asn-207, His-224, Asp-226, His-282 and Phe-298 were verified essential for enzymatic activity. These findings provide insight into structure-function relationship of this pivotal ortho-hydroxylase and also contribute to elucidating the biosynthetic mechanism of coumarin skeleton.
Comparative pharmacokinetic study of pyranocoumarins and khellactone in normal and acute lung injury rats after oral administration of Peucedanum praeruptorum Dunn extracts using a rapid and sensitive liquid chromatography-tandem mass spectrometry method.[Pubmed: 28321891]
Pyranocoumarins are the main constitutes in Peucedanum praeruptorum Dunn and possess various biological activities. In this article, we developed and validated a rapid and sensitive liquid chromatography-tandem mass spectrometry method for the targeted quantification of the pyranocoumarins, praeruptorin A, praeruptorin B and praeruptorin E, and khellactone, which is a common metabolite of these pyranocoumarins in rat plasma samples. We then performed a comparative pharmacokinetic study of these pyranocoumarins and khellactone in normal and lipopolysaccharide-induced acute lung injury (ALI) in rats following oral administration of P. praeruptorum Dunn extracts. Calibration curves gave desirable linearity (r > 0.99) and the lower limit of quantifications were sufficient for quantitative analysis. The precision and accuracy were assessed by intra-batch and inter-batch assays, and the relative standard deviations were all within 10.23% and the accuracy (relative error) was between -5.52% and 8.68%. The extraction recoveries, matrix effects and stability were also acceptable. The pharmacokinetic study revealed that the area under the concentration-time curve (0-t) of khellactone in ALI rats was significantly decreased compared with the normal rats. Meanwhile, the systemic exposures of these pyranocoumarins were slightly higher in the ALI rats than those in normal rats were. The pharmacokinetic study in the pathological state might provide information that was more comprehensive to guide the clinical usage of P. praeruptorum Dunn.
[The inhibition of carboxylesterases by praeruptorin C, D and E].[Pubmed: 29911771]
Praeruptorin C (PC), D (PD) and E (PE) are important compounds extracted from Peucedanum praeruptorum DUNN and have been reported to exert multiple pharmacological activities. The present study is purposed to determine the inhibition of PC, PD and PE on the activity of important phase I metabolic enzymes – carboxylesterases (CES). In vitro human liver microsomes (HLM) incubation system was used to determine the inhibition potential of PC, PD and PE on the activity of CES1 and CES2. Inhibition behaviour was determined, and in vitro-in vivo extrapolation was performed by using the combination of in vitro inhibition kinetic parameter (K(I)) and in vivo exposure level of PD. PD exhibited the strongest inhibition on the activity of CES1, with 81.7% activity inhibited by 100 μmol·L(-1) of PD. PD noncompetitively inhibited the activity of CES1 with the K(I) to be 122.2 μmol·L(-1), indicating inhibition potential of PD towards CES1 in vivo. Therefore, closely monitoring the endogenous metabolic disorders caused by PD and interaction between PD and drugs mainly undergoing CES1-catalyzed metabolism is very necessary.


