Phellodendron chinense
Phellodendron chinense
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
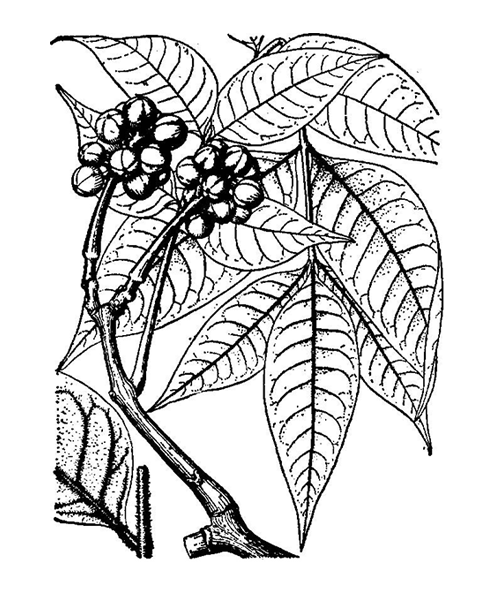
Natural products/compounds from Phellodendron chinense
- Cat.No. Product Name CAS Number COA
-
BCN5934
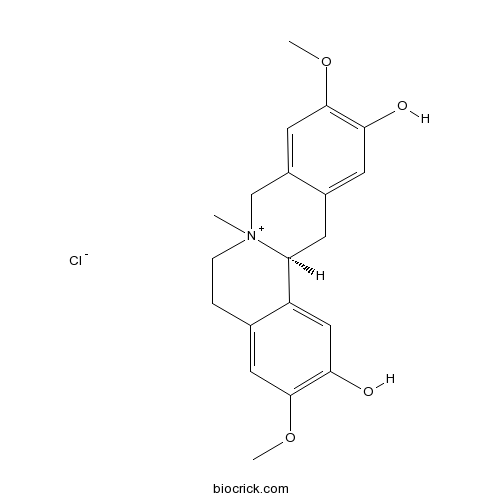
Phellodendrine chloride104112-82-5
Instructions
-
BCN5914
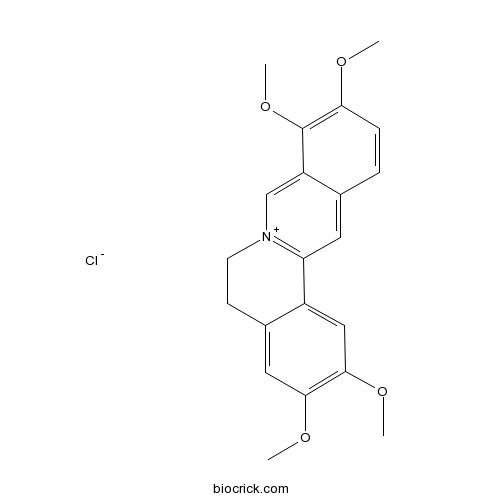
Palmatine hydrochloride10605-02-4
Instructions
-
BCN2651
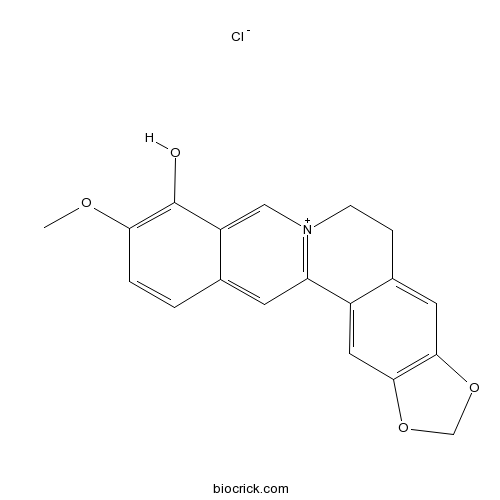
Berberrubine15401-69-1
Instructions
-
BCN2722
Columbamine3621-36-1
Instructions

-
BCN6319
Berberine hydrochloride633-65-8
Instructions
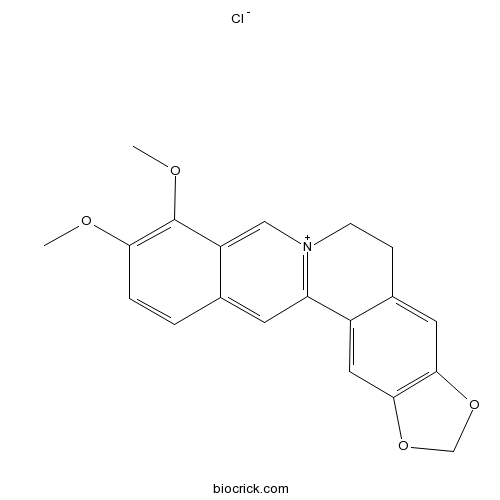
-
BCN5933
Phellodendrine6873-13-8
Instructions
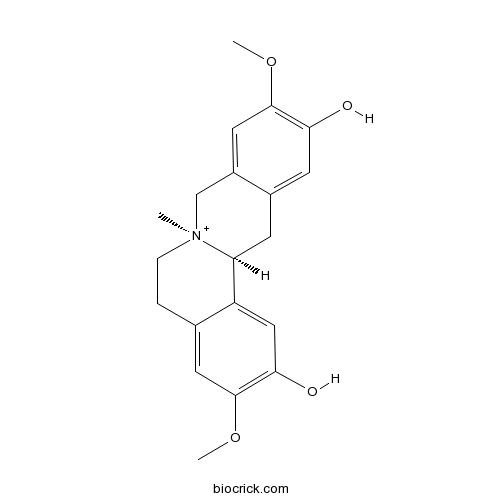
-
BCN4303
Obacunone751-03-1
Instructions

Phellodendron chinense Schneid: A novel yellow-emitting luminescent material for white light-emitting diodes.[Pubmed: 28827629]
None
Phytochemical Quantification and the In Vitro Acetylcholinesterase Inhibitory Activity of Phellodendron chinense and Its Components.[Pubmed: 28574473]
None
Metabolites identification of berberine in rats using ultra-high performance liquid chromatography/quadrupole time-of-flight mass spectrometry.[Pubmed: 28279930]
Berberine (BBR), the principle component for many medicinal plants such as Coptis chinensis Franch., Phellodendron chinense Schneid., and Mahonia bealei (Fort.) Carr., possesses diverse pharmacological activities, including anti-bacterial, anti-inflammatory, antitumor, hypolipidemic and antidiabetic activities. In this study, a rapid and reliable method using a five-step strategy based on the ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (UPLC/Q-TOF-MS), and metabolynx™ software with mass defect filter (MDF) technique was developed to investigate the metabolism of BBR. Plasma, bile, urine and feces samples were collected from rats after oral administration of BBR with a dose of 100mg/kg/day for three consecutive days and analyzed to characterize the metabolic profile of BBR. By comparing the molecular weights and MS fragmentations of the metabolites with those of the parent drug and reference standards, a total of 97 metabolites were identified, including 68 metabolites in urine, 45 metabolites in plasma, 44 metabolites in bile and 41 metabolites in feces. Demethylation, demethylenation, reduction, hydroxylation, and subsequent glucuronidation, sulfation and methylation were the major metabolic pathways of BBR in vivo.
High-throughput screening of triplex DNA binders from complicated samples by 96-well pate format in conjunction with peak area-fading UHPLC-Orbitrap MS.[Pubmed: 28138667]
Conventional strategies for the screening of DNA triplex binders cannot be used for complicated samples, such as ligand libraries created by combinatorial chemistry or from natural product extracts. In the current study, an ultra-high-performance liquid chromatography coupled with an Orbitrap mass spectrometry (UHPLC-Orbitrap-MS)-based approach, which we call peak area-fading (PAF) UHPLC-Orbitrap-MS and was designed for just such a purpose, is reported. The triplex DNA modified 96-well plate and the single stranded oligonucleotide modified 96-well plate (as control) were incubated with ligand libraries, and the unbound ligands were directly determined via UHPLC-ESI-MS. The binders were detected through the decrease (fading) in the peak areas compared to those of the control group. Several factors, such as incubation time, incubation temperature, and buffer, which might affect the binding affinity and reproducibility, were optimized. The potential of the approach was examined using the extracts of Rhizoma Coptidis and Phellodendron chinense Schneid cortexe. The triplex DNA-binding capabilities of the five components (epiberberine, coptisine, jatrorrhizine, berberrubine, and columbamine) were found for the first time, indicating their efficiency for the analysis of complicated samples. In contrast to our previous study, which suffered from a serious drawback of poor reproducibility, this method is more robust and more suitable for high-throughput measurements, opening a new experimental strategy in assessing large libraries of potential drug candidates that work by forming a drug/DNA complex.
Therapeutic effects of Qian-Yu decoction and its three extracts on carrageenan-induced chronic prostatitis/chronic pelvic pain syndrome in rats.[Pubmed: 28122556]
Qian-Yu decoction (QYD) is a traditional Chinese medicinal recipe composed of Radix astragali (Astragalus membranaceus (Fisch.) Bunge var. mongholicus (Bunge) P.K. Hsiao, Fabaceae ), Herba epimedii (Epimedium brevicornum Maxim., Berberidaceae), Herba leonuri (Leonurus japonicus Houtt., Lamiaceae), Cortex phellodendri (Phellodendron chinense Schneid., Rutaceae) and Radix achyranthis bidentatae (Achyranthes bidentata Bl., Amaranthaceae). This study aimed to evaluate the therapeutic activity of QYD against carrageenan-induced chronic prostatic/chronic pelvic pain syndrome (CP/CPPS) in rats and further elucidate its effective components.
Extracts from Traditional Chinese Medicinal Plants Inhibit Acetylcholinesterase, a Known Alzheimer's Disease Target.[Pubmed: 27589716]
Inhibition of acetylcholinesterase (AChE) is a common treatment for early stages of the most general form of dementia, Alzheimer's Disease (AD). In this study, methanol, dichloromethane and aqueous crude extracts from 80 Traditional Chinese Medical (TCM) plants were tested for their in vitro anti-acetylcholinesterase activity based on Ellman's colorimetric assay. All three extracts of Berberis bealei (formerly Mahonia bealei), Coptis chinensis and Phellodendron chinense, which contain numerous isoquinoline alkaloids, substantially inhibited AChE. The methanol and aqueous extracts of Coptis chinensis showed IC50 values of 0.031 µg/mL and 2.5 µg/mL, therefore having an up to 100-fold stronger AChE inhibitory activity than the already known AChE inhibitor galantamine (IC50 = 4.33 µg/mL). Combinations of individual alkaloids berberine, coptisine and palmatine resulted in a synergistic enhancement of ACh inhibition. Therefore, the mode of AChE inhibition of crude extracts of Coptis chinensis, Berberis bealei and Phellodendron chinense is probably due to of this synergism of isoquinoline alkaloids. All extracts were also tested for their cytotoxicity in COS7 cells and none of the most active extracts was cytotoxic at the concentrations which inhibit AChE. Based on these results it can be stated that some TCM plants inhibit AChE via synergistic interaction of their secondary metabolites. The possibility to isolate pure lead compounds from the crude extracts or to administer these as nutraceuticals or as cheap alternative to drugs in third world countries make TCM plants a versatile source of natural inhibitors of AChE.
Berberine Is a Novel Type Efflux Inhibitor Which Attenuates the MexXY-Mediated Aminoglycoside Resistance in Pseudomonas aeruginosa.[Pubmed: 27547203]
The emergence and spread of multidrug-resistant P. aeruginosa infections is of great concern, as very few agents are effective against strains of this species. Methanolic extracts from the Coptidis Rhizoma (the rhizomes of Coptis japonica var. major Satake) or Phellodendri Cortex (the bark of Phellodendron chinense Schneider) markedly reduced resistance to anti-pseudomonal aminoglycosides (e.g., amikacin) in multidrug-resistant P. aeruginosa strains. Berberine, the most abundant benzylisoquinoline alkaloid in the two extracts, reduced aminoglycoside resistance of P. aeruginosa via a mechanism that required the MexXY multidrug efflux system; berberine also reduced aminoglycoside MICs in Achromobacter xylosoxidans and Burkholderia cepacia, two species that harbor intrinsic multidrug efflux systems very similar to the MexXY. Furthermore this compound inhibited MexXY-dependent antibiotic resistance of other classes including cephalosporins (cefepime), macrolides (erythromycin), and lincosamides (lincomycin) demonstrated using a pseudomonad lacking the four other major Mex pumps. Although phenylalanine-arginine beta-naphthylamide (PAβN), a well-known efflux inhibitor, antagonized aminoglycoside in a MexXY-dependent manner, a lower concentration of berberine was sufficient to reduce amikacin resistance of P. aeruginosa in the presence of PAβN. Moreover, berberine enhanced the synergistic effects of amikacin and piperacillin (and vice versa) in multidrug-resistant P. aeruginosa strains. Thus, berberine appears to be a novel type inhibitor of the MexXY-dependent aminoglycoside efflux in P. aeruginosa. As aminoglycosides are molecules of choice to treat severe infections the clinical impact is potentially important.
Tirucallane-type triterpenoids from the fruits of Phellodendron chinense Schneid and their cytotoxic activities.[Pubmed: 27491752]
Eleven triterpenoids were isolated from the fruits of Phellodendron chinense Schneid, and their structures were determined by spectroscopic analysis. The results show that four new tirucallane-type triterpenoids 1, 2, 5, and 6 and seven known compounds 3, 4, 7, 8, 9, 10, and 11 were isolated. Structurally, compound 6 was uncommon; it has a chlorine atom instead of a methyl group at the C-20 position. The cytotoxicities of the compounds was evaluated against the in vitro proliferation of four human tumor cell lines HEL, K562, MDA, and PC3 using adriamycin as the positive control. Compound 1 showed a similar cytotoxicity as the positive control; compounds 3 and 10 showed moderate cytotoxicities compared to the control (P<0.05). This indicates that these compounds have great potential for the development of new antitumor drugs.
DECOCTION PROCESS OPTIMIZATION AND QUALITY EVALUATION OF YI-HUANG DECOCTION BY HPLC FINGERPRINT ANALYSIS.[Pubmed: 29648711]
Yi Huang decoction (YHD) has been used as one of famous traditional formula because of its unique effectiveness against gynecological diseases. YHD is composed of five herbs, including the rootstock of Dioscoma opposita Thunb. (Dioscoreaceae), the kernel of Etayale ferx Salisb. (Nymphaeaceae), the bark of Phellodendron chinense Schneid. (Rutaceae), the seed of Plantago asiatica L. (Plantaginaceae), and the seed of Ginkgo biloba L. (Ginkgoaceae). To effectively control the quality, the processing method for YHD was optimized by means of single factor test as well as orthogonal test in this study. A completely validated method based on HPLC coupled with diode array detector was performed on a Kromasil C(18) column at 30° with mobile phase of 0.1% aqueous phosphoric acid and acetonitrile. As a result, HPLC fingerprint on the basis of the chromatographic data from 32 batches of samples was obtained, which contained 44 common peaks. Among these common peaks, 6 peaks were identified as geniposidic acid, berberine hydrochloride, palmatine hydrochloride, phellodendrine chloride, magnoflorine, and verbascoside, respectively, based on their retention time relative to the standards. Meanwhile, the contents of these 6 compounds were also simultaneously examined. In sum, this study offered valuable information for the proper processing and quality control for YHD.
A Hexa-Herbal TCM Decoction Used to Treat Skin Inflammation: An LC-MS-Based Phytochemical Analysis.[Pubmed: 27272397]
In order to understand the chemical relationship between a traditional hexa-herbal Chinese medicine formula and botanical drugs it is derived from, an analytical platform comprising liquid chromatography coupled with triple quadrupole mass spectrometry and data mining was developed to separate and identify key chemical components. The hexa-herbal formula comprises the rootstock of Scutellaria baicalensis, Rheum tanguticum, Sophora flavescens, the root bark of Dictamnus dasycarpus, the bark of Phellodendron chinense, and the fruit of Kochia scoparia. Seventy-three compounds including alkaloids, anthraquinone derivatives, coumarins, coumarins derivatives, flavonoids, flavone glycosides, naphthalene derivatives, phenylbutanone glucopyranoside, phenolic acids, pterocarpans, stilbenes, stilbenes derivatives, and tannins were putatively identified based on mass measurement and characteristic fragment ions. Among the botanical drugs of the hexa-herbal Chinese medicine formula, the rootstock of R. tanguticum and S. flavescens, bark of P. chinense, and rootstock of S. baicalensis contributed to the majority of the extracted metabolites of the formula decoction. The developed method appeared to be a versatile tool for monitoring chemical constituents in extracts of a traditional Chinese medicine formula in a relatively comprehensive and systematic manner, and helped to understand the importance of the individual botanical drugs within a formulation.


