Phytolacca acinosa
Phytolacca acinosa
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
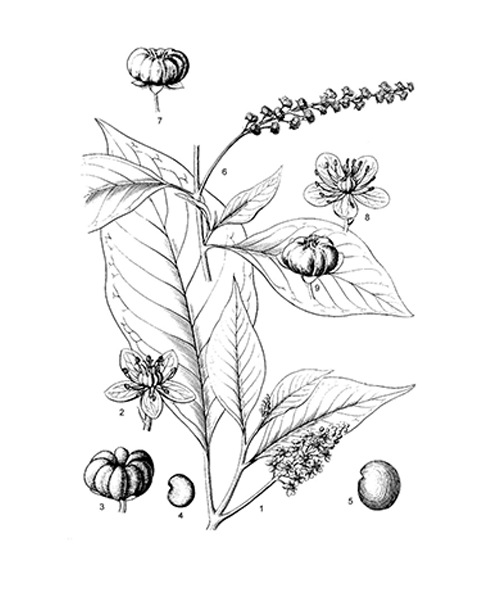
Natural products/compounds from Phytolacca acinosa
- Cat.No. Product Name CAS Number COA
-
BCN1140
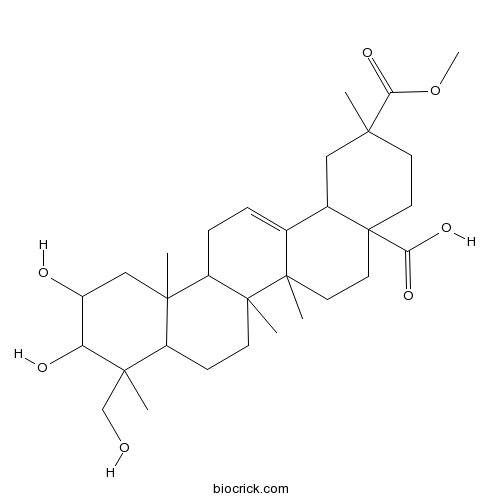
Phytolaccagenin1802-12-6
Instructions
-
BCN5010
Esculentoside A65497-07-6
Instructions

-
BCN2795
Esculentoside H66656-92-6
Instructions

[Triterpenoid saponins from roots of Phytolacca acinosa].[Pubmed: 29950074]
A new triterpenoid saponin named esculentoside U(1), along with the five known compounds, was isolated and characterized from the roots of Phytolacca acinosa, a commonly used traditional Chinese medicine with anti-inflammatory and anti-rheumatoid activities. The structure of the new saponin was elucidated as 3-O-[β-D-glucopyranosyl-(1→4)]-β-D-xylopyranosyl]-2, 23-dihydroxyolean-11, 13(18)-diene-28, 29-dioic acid 29-methyl ester(1). The assignment of all NMR signals of 1 was performed by means of 2D-NMR experiments.
Novel Flavones from the Root of Phytolacca acinosa Roxb.[Pubmed: 28963759]
None
Biochar provides a safe and value-added solution for hyperaccumulating plant disposal: A case study of Phytolacca acinosa Roxb. (Phytolaccaceae).[Pubmed: 28319742]
In this work, an innovative approach using biochar technology for hyperaccumulator disposal was developed and evaluated. The heavy metal enriched P. acinosa biomass (PBM) was pyrolyzed to produce biochar (PBC). Both PBM and PBC were characterized with X-ray diffraction (XRD) for crystal phases, scanning electron microscopy (SEM) for surface topography, and analyzed for elemental composition and mobility. The results revealed that whewellite, a dominant crystal form in biomass, was decomposed to calcite after pyrolysis. Elemental analysis indicated that 91-99% total non-volatile elements in the biomass were retained in the biochar. The toxicity characteristic leaching procedure (TCLP) results revealed that 94.6% and 0.15% of total Mn was extracted for biomass and biochar, respectively. This suggests that mobility and bioavailability of Mn in biochar was much lower relative to pristine biomass. Batch sorption experiment showed that excellent removal of aqueous silver, lead, cadmium, and copper ions can be achieved with PBC. Findings from this work indicated that biochar technology can provide a value-added solution for hyperaccumulator disposal.
Interaction of Fe-Mn plaque and Arthrobacter echigonensis MN1405 and uptake and translocation of Cd by Phytolacca acinosa Roxb.[Pubmed: 28193591]
None
Phytolacca acinosa Roxb. with Arthrobacter echigonensis MN1405 enhances heavy metal phytoremediation.[Pubmed: 27159623]
The growth and metal-extraction efficiency of plants when exposed to toxic metals can be enhanced by inoculating with certain bacteria, but the mechanisms of this process remain unclear. We report results from glasshouse experiments on the effect of Arthrobacter echigonensis MN1405 in promoting Phytolacca acinosa Roxb. growth when exposed to 100 mg/L Mn solution. Mn removal efficiency in solution was significantly enhanced by bacterial inoculation; Mn was accumulated in the root of P. acinosa Roxb. plant. The bacteria oxidized the Mn on root surface, which formed a Mn plaque to serve as a barrier or a containment to prevent metal toxicity. In this process, pH condition was an important factor on the effects of microbial-assisted heavy metal phytoremediation. Our finding suggests that A. echigonensis MN1405 assisted P. acinosa to achieve high remediation efficiency of Mn removal and accumulation in Mn contamination area.
Structural analysis of a type 1 ribosome inactivating protein reveals multiple L‑asparagine‑N‑acetyl‑D‑glucosamine monosaccharide modifications: Implications for cytotoxicity.[Pubmed: 26238506]
Pokeweed antiviral protein (PAP) belongs to the family of type I ribosome‑inactivating proteins (RIPs): Ribotoxins, which function by depurinating the sarcin‑ricin loop of ribosomal RNA. In addition to its antibacterial and antifungal properties, PAP has shown promise in antiviral and targeted tumor therapy owing to its ability to depurinate viral RNA and eukaryotic rRNA. Several PAP genes are differentially expressed across pokeweed tissues, with natively isolated seed forms of PAP exhibiting the greatest cytotoxicity. To help elucidate the molecular basis of increased cytotoxicity of PAP isoenzymes from seeds, the present study used protein sequencing, mass spectroscopy and X-ray crystallography to determine the complete covalent structure and 1.7 Å X‑ray crystal structure of PAP‑S1aci isolated from seeds of Asian pokeweed (Phytolacca acinosa). PAP‑S1aci shares ~95% sequence identity with PAP‑S1 from P. americana and contains the signature catalytic residues of the RIP superfamily, corresponding to Tyr72, Tyr122, Glu175 and Arg178 in PAP‑S1aci. A rare proline substitution (Pro174) was identified in the active site of PAP‑S1aci, which has no effect on catalytic Glu175 positioning or overall active‑site topology, yet appears to come at the expense of strained main‑chain geometry at the pre‑proline residue Val173. Notably, a rare type of N‑glycosylation was detected consisting of N‑acetyl‑D‑glucosamine monosaccharide residues linked to Asn10, Asn44 and Asn255 of PAP‑S1aci. Of note, our modeling studies suggested that the ribosome depurination activity of seed PAPs would be adversely affected by the N‑glycosylation of Asn44 and Asn255 with larger and more typical oligosaccharide chains, as they would shield the rRNA‑binding sites on the protein. These results, coupled with evidence gathered from the literature, suggest that this type of minimal N‑glycosylation in seed PAPs and other type I seed RIPs may serve to enhance cytotoxicity by exploiting receptor‑mediated uptake pathways of seed predators while preserving ribosome affinity and rRNA recognition.


