PhytolaccageninCAS# 1802-12-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1802-12-6 | SDF | Download SDF |
| PubChem ID | 272184 | Appearance | White powder |
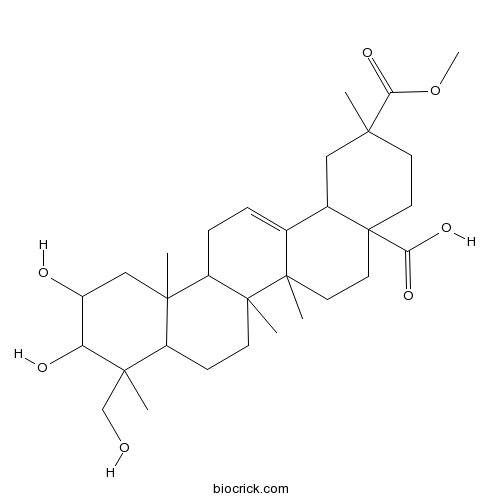
| Formula | C31H48O7 | M.Wt | 532.7 |
| Type of Compound | Triterpenoids | Storage | Desiccate at -20°C |
| Synonyms | Jaligonic acid 30-methyl ester | ||
| Solubility | Soluble in methan | ||
| Chemical Name | 10,11-dihydroxy-9-(hydroxymethyl)-2-methoxycarbonyl-2,6a,6b,9,12a-pentamethyl-1,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydropicene-4a-carboxylic acid | ||
| SMILES | CC1(CCC2(CCC3(C(=CCC4C3(CCC5C4(CC(C(C5(C)CO)O)O)C)C)C2C1)C)C(=O)O)C(=O)OC | ||
| Standard InChIKey | CYJWWQALTIKOAG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C31H48O7/c1-26(25(37)38-6)11-13-31(24(35)36)14-12-29(4)18(19(31)15-26)7-8-22-27(2)16-20(33)23(34)28(3,17-32)21(27)9-10-30(22,29)5/h7,19-23,32-34H,8-17H2,1-6H3,(H,35,36) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Phytolaccagenin has promising antifungal activity against ATCC standard cultures of Candida albicans and Cryptococcus neoformans, and against clinical isolates of these fungi, it also shows inhibitory effects on lipopolysaccharide-induced NO production, and haemolytic activities. |
| Targets | Antifection | NO |
| In vitro | Antifungal activity of saponin-rich extracts of Phytolacca dioica and of the sapogenins obtained through hydrolysis.[Pubmed: 20734930]Nat. Prod. Commun.,2010, 5(7):1013-8.
|
| Structure Identification | J Pharm Biomed Anal. 2015 Mar 25;107:82-8.Development and validation of a HPLC-MS/MS method for the determination of phytolaccagenin in rat plasma and application to a pharmacokinetic study.[Pubmed: 25575173]Radix Phytolaccae (the dried root of Phytolacca acinosa Roxb. or Phytolacca americana L.) is widely used in East Asian countries for the treatment of inflammation-related diseases.
The active component of Radix Phtolaccae is Phytolcaccagenin a triterpenoid saponin. Phytolcaccagenin has anti-inflammatory activities that exceed those of Esculentoside A and its derivatives regarding suppression of LPS-induced inflammation, and has a lower toxicity profile with less hemolysis. To date, no information is available about analytical method and pharmacokinetic studies of Phytolaccagenin.
|

Phytolaccagenin Dilution Calculator

Phytolaccagenin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8772 mL | 9.3861 mL | 18.7723 mL | 37.5446 mL | 46.9307 mL |
| 5 mM | 0.3754 mL | 1.8772 mL | 3.7545 mL | 7.5089 mL | 9.3861 mL |
| 10 mM | 0.1877 mL | 0.9386 mL | 1.8772 mL | 3.7545 mL | 4.6931 mL |
| 50 mM | 0.0375 mL | 0.1877 mL | 0.3754 mL | 0.7509 mL | 0.9386 mL |
| 100 mM | 0.0188 mL | 0.0939 mL | 0.1877 mL | 0.3754 mL | 0.4693 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ganoderlactone D
Catalog No.:BCN7849
CAS No.:1801934-15-5
- Anthracophyllone
Catalog No.:BCN7606
CAS No.:1801750-22-0
- Megastigm-7-ene-3,4,6,9-tetrol
Catalog No.:BCN6511
CAS No.:180164-14-1
- Quercetin 3-O-glucoside-7-O-rhamnoside
Catalog No.:BCN1520
CAS No.:18016-58-5
- Bupivacaine HCl
Catalog No.:BCC4406
CAS No.:18010-40-7
- SB 224289 hydrochloride
Catalog No.:BCC6976
CAS No.:180084-26-8
- Voafinidine
Catalog No.:BCN6738
CAS No.:180059-77-2
- SDZ NKT 343
Catalog No.:BCC7349
CAS No.:180046-99-5
- Ketohakonanol
Catalog No.:BCN7427
CAS No.:18004-20-1
- 7-Chlorokynurenic acid
Catalog No.:BCC6577
CAS No.:18000-24-3
- 3-oxo-Olean-12-en-28-oic acid
Catalog No.:BCN3171
CAS No.:17990-42-0
- 27-Hydroxymangiferolic acid
Catalog No.:BCN4689
CAS No.:17983-82-3
- NCT-501
Catalog No.:BCC6539
CAS No.:1802088-50-1
- Cyclo(Leu-Ala)
Catalog No.:BCN2428
CAS No.:1803-60-7
- Perillaldehyde
Catalog No.:BCN8294
CAS No.:18031-40-8
- Solifenacin hydrochloride
Catalog No.:BCC5193
CAS No.:180468-39-7
- Fmoc-D-Asn(Trt)-OH
Catalog No.:BCC3084
CAS No.:180570-71-2
- Gentioflavin
Catalog No.:BCN3619
CAS No.:18058-50-9
- Peiminine
Catalog No.:BCN1095
CAS No.:18059-10-4
- 2,2'-Biphenol
Catalog No.:BCC8488
CAS No.:1806-29-7
- 1,4-Bis(5-phenyl-2-oxazolyl)benzene
Catalog No.:BCC8424
CAS No.:1806-34-4
- Polypodine B
Catalog No.:BCN8117
CAS No.:18069-14-2
- LG 100754
Catalog No.:BCC7786
CAS No.:180713-37-5
- CHMFL-ABL-053
Catalog No.:BCC3988
CAS No.:1808287-83-3
Antifungal activity of saponin-rich extracts of Phytolacca dioica and of the sapogenins obtained through hydrolysis.[Pubmed:20734930]
Nat Prod Commun. 2010 Jul;5(7):1013-8.
A saponin-rich extract of Phytolacca dioica L. berries, its acid hydrolysate, and its major aglycone, Phytolaccagenin, were assayed for antifungal activity against ATCC standard cultures of Candida albicans and Cryptococcus neoformans, and against clinical isolates of these fungi. The activity of the extract was either low or negligible, but the hydrolysate, containing the sapogenins, including Phytolaccagenin, and also pure Phytolaccagenin, showed promising antifungal potency. Hydrolysis of a natural product extract is shown to be a useful modification leading to improved bioactivity.
Development and validation of a HPLC-MS/MS method for the determination of phytolaccagenin in rat plasma and application to a pharmacokinetic study.[Pubmed:25575173]
J Pharm Biomed Anal. 2015 Mar 25;107:82-8.
Radix Phytolaccae (the dried root of Phytolacca acinosa Roxb. or Phytolacca americana L.) is widely used in East Asian countries for the treatment of inflammation-related diseases. The active component of Radix Phtolaccae is Phytolcaccagenin a triterpenoid saponin. Phytolcaccagenin has anti-inflammatory activities that exceed those of Esculentoside A and its derivatives regarding suppression of LPS-induced inflammation, and has a lower toxicity profile with less hemolysis. To date, no information is available about analytical method and pharmacokinetic studies of Phytolaccagenin. To explore PK profile of this compound, a HPLC-MS/MS assay of Phytolaccagenin in rat plasma was developed and validated. The method was fully validated according to FDA Guidance for industry. The detection was performed by a triple-quadrupole tandem mass spectrometer with multiple reactions monitoring (MRM) in positive ion mode via electrospray ionization. The monitored transitions were m/z 533.2>515.3 for Phytolcaccagenin, and 491.2>473.2 for I.S. The analysis was performed on a Symmetry C18 column (4.6 mm x 50 mm, 3.5 mum) using gradient elution with the mobile phase consisting of acetonitrile and 0.1% formic acid water at a flow rate of 1 ml/min with a 1:1 splitter ratio. The method was validated with a LLOQ of 20 ng/ml and an ULOQ of 1000 ng/ml. The response versus concentration data were fitted with 1/x weighting and the correlation coefficient (r) were greater than 0.999. The average matrix effect and the average extraction recovery were acceptable. This validation in rat plasma demonstrated that Phytolaccagenin was stable for 30 days when stored below -20 degrees C, for 6h at room temperature (RT, 22 degrees C), for 12 h at RT for prepared control samples in auto-sampler vials, and during three successive freeze/thaw cycles results at -20 degrees C. The validated method has been successfully applied to an intravenous bolus pharmacokinetic study of Phytolaccagenin in male Sprague-Dawley rats (10 mg/kg, i.v.). Blood samples taken from 0 to 24h after injection were collected, and data analyzed with WinNonlin. The half-life and clearance were 1.4+/-0.9 h and 2.1+/-1.1 L/h/kg, respectively.
Synthesis of novel derivatives of esculentoside A and its aglycone phytolaccagenin, and evaluation of their haemolytic activity and inhibition of lipopolysaccharide-induced nitric oxide production.[Pubmed:22006712]
Chem Biodivers. 2011 Oct;8(10):1833-52.
A series of 46 compounds derived from esculentoside A and its aglycone were synthesized and characterized. The effect of these compounds on lipopolysaccharide (LPS)-induced NO production, haemolytic activity, and cell viability was evaluated. Structure-activity relationship was established by comparing the derivatives of esculentoside A with its aglycone derivatives. Both the aglycone and its derivatives showed higher inhibitory effects on LPS-induced NO production, and lower haemolytic activities than esculentoside A and its derivatives.


