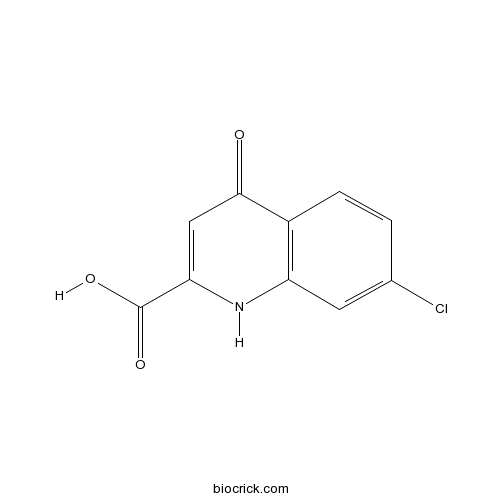
7-Chlorokynurenic acidPotent competitive inhibitor of L-glutamate uptake CAS# 18000-24-3 |
- NSC 23766
Catalog No.:BCC1149
CAS No.:1177865-17-6
- Zoledronic Acid
Catalog No.:BCC1067
CAS No.:118072-93-8
- EHop-016
Catalog No.:BCC5022
CAS No.:1380432-32-5
- ZCL278
Catalog No.:BCC3665
CAS No.:587841-73-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 18000-24-3 | SDF | Download SDF |
| PubChem ID | 1884 | Appearance | Powder |
| Formula | C10H6ClNO3 | M.Wt | 223.62 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 16.67 mg/mL (74.55 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | 7-chloro-4-oxo-1H-quinoline-2-carboxylic acid | ||
| SMILES | C1=CC2=C(C=C1Cl)NC(=CC2=O)C(=O)O | ||
| Standard InChIKey | UAWVRVFHMOSAPU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H6ClNO3/c11-5-1-2-6-7(3-5)12-8(10(14)15)4-9(6)13/h1-4H,(H,12,13)(H,14,15) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | NMDA receptor antagonist acting at the glycine site. Potent competitive inhibitor of L-glutamate transport into synaptic vesicles. Also available as part of the NMDA Receptor - Glycine Site. 7-Chlorokynurenic acid sodium salt also available. |

7-Chlorokynurenic acid Dilution Calculator

7-Chlorokynurenic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.4719 mL | 22.3594 mL | 44.7187 mL | 89.4374 mL | 111.7968 mL |
| 5 mM | 0.8944 mL | 4.4719 mL | 8.9437 mL | 17.8875 mL | 22.3594 mL |
| 10 mM | 0.4472 mL | 2.2359 mL | 4.4719 mL | 8.9437 mL | 11.1797 mL |
| 50 mM | 0.0894 mL | 0.4472 mL | 0.8944 mL | 1.7887 mL | 2.2359 mL |
| 100 mM | 0.0447 mL | 0.2236 mL | 0.4472 mL | 0.8944 mL | 1.118 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 3-oxo-Olean-12-en-28-oic acid
Catalog No.:BCN3171
CAS No.:17990-42-0
- 27-Hydroxymangiferolic acid
Catalog No.:BCN4689
CAS No.:17983-82-3
- Aflastatin A
Catalog No.:BCN1822
CAS No.:179729-59-0
- N-Benzoyl-leucine
Catalog No.:BCC9092
CAS No.:17966-67-5
- Macrocarpal J
Catalog No.:BCN1139
CAS No.:179603-47-5
- Amabiline
Catalog No.:BCN1950
CAS No.:17958-43-9
- Cynaustine
Catalog No.:BCN1951
CAS No.:17958-39-3
- Cynaustraline
Catalog No.:BCN2048
CAS No.:17958-37-1
- PD 150606
Catalog No.:BCC2353
CAS No.:179528-45-1
- 1,2,3,4-Tetrahydronorharman-1-one
Catalog No.:BCN3690
CAS No.:17952-82-8
- Venoterpine
Catalog No.:BCN3422
CAS No.:17948-42-4
- Rutacridone
Catalog No.:BCN7542
CAS No.:17948-33-3
- Ketohakonanol
Catalog No.:BCN7427
CAS No.:18004-20-1
- SDZ NKT 343
Catalog No.:BCC7349
CAS No.:180046-99-5
- Voafinidine
Catalog No.:BCN6738
CAS No.:180059-77-2
- SB 224289 hydrochloride
Catalog No.:BCC6976
CAS No.:180084-26-8
- Bupivacaine HCl
Catalog No.:BCC4406
CAS No.:18010-40-7
- Quercetin 3-O-glucoside-7-O-rhamnoside
Catalog No.:BCN1520
CAS No.:18016-58-5
- Megastigm-7-ene-3,4,6,9-tetrol
Catalog No.:BCN6511
CAS No.:180164-14-1
- Anthracophyllone
Catalog No.:BCN7606
CAS No.:1801750-22-0
- Ganoderlactone D
Catalog No.:BCN7849
CAS No.:1801934-15-5
- Phytolaccagenin
Catalog No.:BCN1140
CAS No.:1802-12-6
- NCT-501
Catalog No.:BCC6539
CAS No.:1802088-50-1
- Cyclo(Leu-Ala)
Catalog No.:BCN2428
CAS No.:1803-60-7
7-Chlorokynurenic acid (7-CTKA) produces rapid antidepressant-like effects: through regulating hippocampal microRNA expressions involved in TrkB-ERK/Akt signaling pathways in mice exposed to chronic unpredictable mild stress.[Pubmed:25034119]
Psychopharmacology (Berl). 2015 Feb;232(3):541-50.
RATIONALE: 7-Chlorokynurenic acid (7-CTKA), a NMDA receptor antagonist, has been reported as a potential rapid antidepressant with poor understanding about the molecular mechanism of its therapeutic action. MicroRNAs (miRNAs) are emerging as critical regulators of central nervous system plasticity and may play an important role in depression. OBJECTIVE: The objective of this study was to investigate the molecular mechanism of antidepressant action of 7-CTKA in chronic unpredictable mild stress (CUMS) animal model. METHODS: K252a (tropomyosin-related kinase receptor B (TrkB) antagonist), U0126 (extracellular signal-regulated kinase (ERK) phosphorylation inhibitor), LY294002 (serine-threonine kinase (Akt) phosphorylation inhibitor), or vehicle was given intracerebroventricularly to mice in each group 30 min before 7-CTKA or vehicle intraperitoneal injection. Behavioral changes were observed by sucrose preference test and miRNA microarray was performed to examine hippocampal miRNAs levels in mice. Quantitative RT-PCR was conducted to further confirm results in microarray study. RESULTS: 7-CTKA not only reversed the decrease in sucrose preference and multiple hippocampal miRNAs changes induced by CUMS but also mediated 15 common miRNAs via TrkB-ERK/Akt pathways. Among them, the expression levels of four miRNAs (miR-34a-5p, miR-200a-3p, miR-144-3p, miR-1894-5p) were validated by quantitative real-time PCR (qRT-PCR). The findings from qRT-PCR study support results from microarray analysis except for the non-significance of miR-1894-5p expression. CONCLUSIONS: This demonstrated that the 15 miRNA targets shared by TrkB-ERK/Akt pathways might participate in rapid-acting molecular mechanism of antidepressant 7-CTKA.
Activation of hippocampal BDNF signaling is involved in the antidepressant-like effect of the NMDA receptor antagonist 7-chlorokynurenic acid.[Pubmed:26562663]
Brain Res. 2016 Jan 1;1630:73-82.
Previous studies showed that acute 7-Chlorokynurenic acid treatment produced a rapid antidepressant-like action in depression-like animal models. However, the underlying mechanism involved in neurotrophin system about 7-Chlorokynurenic acid is unclear. Our present study aimed to verify whether chronic 7-Chlorokynurenic acid treatment produced an antidepressant-like effect through the activation of brain-derived neurotrophic factor (BDNF) signaling in mice exposed to chronic unpredictable mild stress (CUMS). In addition, we performed an oral toxicological evaluation of chronic 7-Chlorokynurenic acid administration in mice. The results showed that a two-week administration with 7-Chlorokynurenic acid reversed the decreased sucrose preference and prolonged first feeding latency. In addition, 7-Chlorokynurenic acid significantly reversed the CUMS-induced down-regulation of BDNF, p-ERK, p-Akt, PSD-95, synapsin I and cell proliferation in the hippocampus. In contrast, K252a, an inhibitor of BDNF receptor tropomyosin-related kinase receptor B (TrkB), blocked the antidepressant-like effect and the improvement of 7-Chlorokynurenic acid. Furthermore, we found that 7-Chlorokynurenic acid did not produce any toxicological effect in mice. In conclusion, our findings suggest that the antidepressant-like effect of 7-Chlorokynurenic acid may be mediated, at least in part, by activating BDNF signaling in the hippocampus.
Ameliorative effects of histamine on 7-chlorokynurenic acid-induced spatial memory deficits in rats.[Pubmed:12601505]
Psychopharmacology (Berl). 2003 Apr;166(4):360-5.
RATIONALE: Histamine plays an important role in modulating acquisition and retention in learning and memory process in experimental animals. OBJECTIVES: We examined the effects of polyamine and histamine on the N-methyl- d-aspartate (NMDA) receptor glycine site antagonist 7-Chlorokynurenic acid-induced spatial memory deficits in radial maze performance in rats. METHOD: Effects of histamine (0.5 or 1 nmol/site intracerebroventricularly), spermidine (1 nmol/site, intracerebroventricularly) and spermine (1 nmol/site, intracerebroventricularly) on spatial memory deficit in 9-week-old-male Wistar rats were observed. Both reference and working memory errors occurred in radial maze performance in rats, following intracerebroventricular injection of 7-Chlorokynurenic acid (10 nmol/site). RESULTS: Spermidine (1 nmol/site, intracerebroventricularly) or spermine (1 nmol/site, intracerebroventricularly) antagonized 7-Chlorokynurenic acid-induced deficits on working memory but not on reference memory errors. Intracerebroventricular histamine (0.5 or 1 nmol/site) or thioperamide (100 nmol/site) also ameliorated 7-Chlorokynurenic acid-induced working memory deficits. To determine whether the effects of histamine involve histamine receptors, the effects of some methylhistamines were examined. The effects of R-alpha-methylhistamine on radial maze performance were mimicked by histamine. N(alpha)-methylhistamine had no effect on 7-Chlorokynurenic acid-induced memory deficits, whereas 1-methylhistamine, but not 3-methylhistamine reversed 7-Chlorokynurenic acid-induced working memory deficits. CONCLUSION: These results suggest that the amelioration of 7-Chlorokynurenic acid-induced working memory deficits by histamine may involve a direct action of histamine at the polyamine sites on NMDA receptors.
Synthesis, pharmacokinetics and anticonvulsant activity of 7-chlorokynurenic acid prodrugs.[Pubmed:10915929]
Int J Pharm. 2000 Jul 20;202(1-2):79-88.
7-Chlorokynurenic acid 1 is a potent glycine-N-methyl-D-aspartate (NMDA) receptor antagonist, but it shows weak activity after systemic administration. In order to overcome the Blood-brain barrier (BBB), we synthetized three new esters 2-4 of 1 obtained by chemical conjugation with essential nutrients such as glucose and galactose, that are actively transported across the BBB. These compounds were assayed to evaluate their in vitro chemical and enzymatic hydrolysis. In addition the prodrugs 2-4 were tested for their ability to protect mice against NMDA-induced seizures after systemic administration. All the prodrugs 2-4 appeared moderately stable in pH 7.4 buffered solution and were susceptible to in vitro enzymatic hydrolysis. Intraperitoneal administration of either esters 2 or 4 was highly protective against seizures induced by NMDA in mice, with the latter prodrug showing the highest anticonvulsive activity. In addition, ester 4 undergoes a time-dependent extracellular hydrolysis into 1 when applied to mixed cultures of mouse cortical cells, a model that reproduces in vitro the cellular milieu encountered by the prodrugs once they penetrate the brain parenchyma.
Substituted quinolines as inhibitors of L-glutamate transport into synaptic vesicles.[Pubmed:9776380]
Neuropharmacology. 1998 Jul;37(7):839-46.
This study investigated the structure-activity relationships and kinetic properties of a library of kynurenate analogues as inhibitors of 3H-L-glutamate transport into rat forebrain synaptic vesicles. The lack of inhibitory activity observed with the majority of the monocyclic pyridine derivatives suggested that the second aromatic ring of the quinoline-based compounds played a significant role in binding to the transporter. A total of two kynurenate derivatives, xanthurenate and 7-chloro-kynurenate, differing only in the carbocyclic ring substituents, were identified as potent competitive inhibitors, exhibiting Ki values of 0.19 and 0.59 mM, respectively. The Km value for L-glutamate was found to be 2.46 mM. Parallel experiments demonstrated that while none of the kynurenate analogues tested effectively inhibited the synaptosomal transport of 3H-D-aspartate, some cross-reactivity was observed with the EAA ionotropic receptors. Molecular modeling studies were carried out with the identified inhibitors and glutamate in an attempt to preliminarily define the pharmacophore of the vesicular transporter. It is hypothesized that the ability of the kynurenate analogues to bind to the transporter may be tied to the capacity of the quinoline carbocyclic ring to mimic the negative charge of the gamma-carboxylate of glutamate. A total of two low energy solution conformers of glutamate were identified that exhibited marked functional group overlap with the most potent inhibitor, xanthurenate. These results help to further refine the pharmacological specificity of the glutamate binding site on the vesicular transporter and identify a series of inhibitors with which to investigate transporter function.
Behavioral and neurochemical actions of the strychnine-insensitive glycine receptor antagonist, 7-chlorokynurenate, in rats.[Pubmed:7498252]
Eur J Pharmacol. 1995 Jun 23;280(1):37-45.
The present study investigated if blockade of the modulatory glycine receptor of the NMDA receptor complex influences the expression of behavior (sniffing stereotypy and locomotion) and dopamine metabolism in rats as it has been shown for NMDA receptor antagonists. The glycine receptor antagonist, 7-chlorokynurenate (7-chloro-4-hydroxyquinoline-2-carboxylic acid), induced a dose-dependent sniffing stereotypy but had no effect on locomotion when it was given i.c.v. The glycine receptor agonist, D-cycloserine (D-4-amino-3-isoxazolidinone), antagonized the sniffing stereotypy. 7-Chlorokynurenate had no influence on dopamine metabolism in the striatum and the nucleus accumbens, but moderately decreased the metabolism in the prefrontal cortex. Comparison of behavioral and neurochemical outcomes suggests that the failure to induce locomotion correlates with the unchanged dopamine metabolism in the basal ganglia, while sniffing stereotypy does not. These results show that blockade of the glycine receptor of the NMDA receptor complex induces a behavioral and neurochemical profile similar to that of competitive NMDA receptor antagonists.
7-Chlorokynurenic acid is a selective antagonist at the glycine modulatory site of the N-methyl-D-aspartate receptor complex.[Pubmed:2842779]
Proc Natl Acad Sci U S A. 1988 Sep;85(17):6547-50.
Glycine markedly potentiates N-methyl-D-aspartate (N-Me-D-Asp) responses in mammalian neurons by an action at a modulatory site on the N-Me-D-Asp receptor-ionophore complex. Here we present evidence that 7-Chlorokynurenic acid (7-Cl KYNA) inhibits N-Me-D-Asp responses by a selective antagonism of glycine at this modulatory site. In rat cortical slices 7-Cl KYNA (10-100 microM) noncompetitively inhibited N-Me-D-Asp responses, and this effect could be reversed by the addition of glycine (100 microM) or D-serine (100 microM). Radioligand binding experiments showed that 7-Cl KYNA had a much higher affinity for the strychnine-insensitive [3H]glycine binding site (IC50 = 0.56 microM) than for the N-Me-D-Asp (IC50 169 microM), quisqualate (IC50 = 153 microM), or kainate (IC50 greater than 1000 microM) recognition sites. In whole-cell patch-clamp recordings from rat cortical neurones in culture, the inhibitory effects of 7-Cl KYNA on N-Me-D-Asp-induced currents could not be overcome by increasing the N-Me-D-Asp concentration but could be reversed by increasing the glycine concentration. 7-Cl KYNA could completely abolish N-Me-D-Asp responses, including basal responses in the absence of added glycine, suggesting that it may possess negative modulatory effects at the glycine site. These findings indicate that the glycine modulatory site is functional in intact adult tissue and that 7-Cl KYNA should prove to be a selective tool for elucidating the involvement of this site in physiological and pathological events mediated by N-Me-D-Asp receptors.


