LG 100754RXR:PPAR agonist CAS# 180713-37-5 |
- Leucovorin Calcium
Catalog No.:BCC1198
CAS No.:6035-45-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 180713-37-5 | SDF | Download SDF |
| PubChem ID | 6442223 | Appearance | Powder |
| Formula | C26H36O3 | M.Wt | 396.56 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 50 mM in ethanol | ||
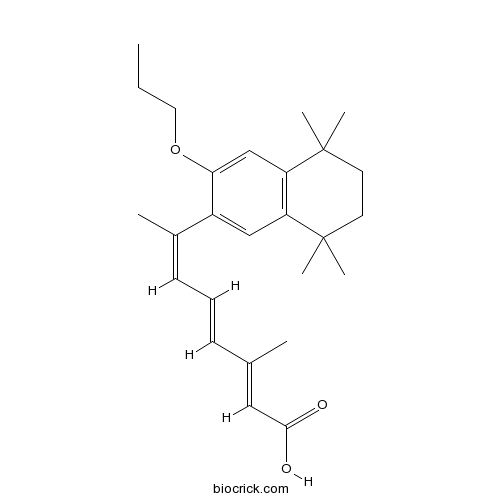
| Chemical Name | (2E,4E,6Z)-3-methyl-7-(5,5,8,8-tetramethyl-3-propoxy-6,7-dihydronaphthalen-2-yl)octa-2,4,6-trienoic acid | ||
| SMILES | CCCOC1=CC2=C(C=C1C(=CC=CC(=CC(=O)O)C)C)C(CCC2(C)C)(C)C | ||
| Standard InChIKey | HNODNXQAYXJFMQ-LQUSFLDPSA-N | ||
| Standard InChI | InChI=1S/C26H36O3/c1-8-14-29-23-17-22-21(25(4,5)12-13-26(22,6)7)16-20(23)19(3)11-9-10-18(2)15-24(27)28/h9-11,15-17H,8,12-14H2,1-7H3,(H,27,28)/b10-9+,18-15+,19-11- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Novel RXR:PPARγ agonist; sensitizes PPARγ by enhancing its ligand binding activity. Also activates RXR:RAR and RXR:PPARα heterodimers in cotransfection assays. Displays selectivity over other permissive heterodimers such as RXR:LXRα and RXR:BAR/FXR. Exhibits antidiabetic properties in vivo. |

LG 100754 Dilution Calculator

LG 100754 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5217 mL | 12.6084 mL | 25.2169 mL | 50.4337 mL | 63.0422 mL |
| 5 mM | 0.5043 mL | 2.5217 mL | 5.0434 mL | 10.0867 mL | 12.6084 mL |
| 10 mM | 0.2522 mL | 1.2608 mL | 2.5217 mL | 5.0434 mL | 6.3042 mL |
| 50 mM | 0.0504 mL | 0.2522 mL | 0.5043 mL | 1.0087 mL | 1.2608 mL |
| 100 mM | 0.0252 mL | 0.1261 mL | 0.2522 mL | 0.5043 mL | 0.6304 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Polypodine B
Catalog No.:BCN8117
CAS No.:18069-14-2
- 1,4-Bis(5-phenyl-2-oxazolyl)benzene
Catalog No.:BCC8424
CAS No.:1806-34-4
- 2,2'-Biphenol
Catalog No.:BCC8488
CAS No.:1806-29-7
- Peiminine
Catalog No.:BCN1095
CAS No.:18059-10-4
- Gentioflavin
Catalog No.:BCN3619
CAS No.:18058-50-9
- Fmoc-D-Asn(Trt)-OH
Catalog No.:BCC3084
CAS No.:180570-71-2
- Solifenacin hydrochloride
Catalog No.:BCC5193
CAS No.:180468-39-7
- Perillaldehyde
Catalog No.:BCN8294
CAS No.:18031-40-8
- Cyclo(Leu-Ala)
Catalog No.:BCN2428
CAS No.:1803-60-7
- NCT-501
Catalog No.:BCC6539
CAS No.:1802088-50-1
- Phytolaccagenin
Catalog No.:BCN1140
CAS No.:1802-12-6
- Ganoderlactone D
Catalog No.:BCN7849
CAS No.:1801934-15-5
- CHMFL-ABL-053
Catalog No.:BCC3988
CAS No.:1808287-83-3
- 4-O-Caffeoylshikimic acid
Catalog No.:BCN7931
CAS No.:180842-65-3
- Jaceosidin
Catalog No.:BCN2529
CAS No.:18085-97-7
- Spiramilactone B
Catalog No.:BCN1141
CAS No.:180961-65-3
- Neuchromenin
Catalog No.:BCN7449
CAS No.:180964-26-5
- FTI 277 HCl
Catalog No.:BCC6395
CAS No.:180977-34-8
- 3-O-Caffeoylshikimic acid
Catalog No.:BCN7930
CAS No.:180981-12-8
- Curcumaromin A
Catalog No.:BCN7417
CAS No.:1810034-38-8
- Curcumaromin B
Catalog No.:BCN7419
CAS No.:1810034-39-9
- Curcumaromin C
Catalog No.:BCN7418
CAS No.:1810034-40-2
- Corymbosin
Catalog No.:BCN6812
CAS No.:18103-41-8
- 5,7-Dihydroxy-3',4',5'-trimethoxyflavone
Catalog No.:BCN6807
CAS No.:18103-42-9
Transferable Reactive Force Fields: Extensions of ReaxFF-lg to Nitromethane.[Pubmed:28177629]
J Phys Chem A. 2017 Mar 9;121(9):2001-2013.
Transferable ReaxFF-lg models of nitromethane that predict a variety of material properties over a wide range of thermodynamic states are obtained by screening a library of approximately 6600 potentials that were previously optimized through the Multiple Objective Evolutionary Strategies (MOES) approach using a training set that included information for other energetic materials composed of carbon, hydrogen, nitrogen, and oxygen. Models that best match experimental nitromethane lattice constants at 4.2 K and 1 atm are evaluated for transferability to high-pressure states at room temperature and are shown to better predict various liquid- and solid-phase structural, thermodynamic, and transport properties as compared to the existing ReaxFF and ReaxFF-lg parametrizations. Although demonstrated for an energetic material, the library of ReaxFF-lg models is supplied to the scientific community to enable new research explorations of complex reactive phenomena in a variety of materials research applications.
Loading of halloysite nanotubes with BSA, alpha-Lac and beta-Lg: a Fourier transform infrared spectroscopic and thermogravimetric study.[Pubmed:28029112]
Nanotechnology. 2017 Feb 3;28(5):055706.
Halloysite nanotubes (HNTs) are considered as ideal materials for biotechnological and medical applications. An important feature of halloysite is that it has a different surface chemistry on the inner and outer sides of the tubes. This property means that negatively-charged molecules can be selectively loaded inside the halloysite nanoscale its lumen. Loaded HNTs can be used for the controlled or sustained release of proteins, drugs, bioactive molecules and other agents. We studied the interaction between HNTs and bovine serum albumin, alpha lactalbumin and beta -lactoglobulin loaded into HTNs using Fourier transform infrared spectroscopy and thermogravimetry. These techniques enabled us to study the protein conformation and thermal stability, respectively, and to estimate the amount of protein loaded into the HNTs. TEM images confirmed the loading of proteins into HTNs.
A GLABRA1 ortholog on LG A9 controls trichome number in the Japanese leafy vegetables Mizuna and Mibuna (Brassica rapa L. subsp. nipposinica L. H. Bailey): evidence from QTL analysis.[Pubmed:28258381]
J Plant Res. 2017 May;130(3):539-550.
Brassica rapa show a wide range of morphological variations. In particular, the leaf morphologies of the Japanese traditional leafy vegetables Mizuna and Mibuna (Brassica rapa L. subsp. nipposinica L. H. Bailey) are distinctly different, even though they are closely related cultivars that are easy to cross. In addition to the differences in the gross morphology of leaves, some cultivars of Mibuna (Kyo-nishiki) have many trichomes on its leaves, whereas Mizuna (Kyo-mizore) does not. To identify the genes responsible for the different number of trichomes, we performed a quantitative trait loci (QTL) analysis of Mizuna and Mibuna. To construct linkage maps for these cultivars, we used RNA-seq data to develop cleaved amplified polymorphic sequence (CAPS) markers. We also performed a restriction site-associated DNA sequencing (RAD-seq) analysis to detect single-nucleotide polymorphisms (SNPs). Two QTL analyses were performed in different years, and both analyses indicated that the largest effect was found on LG A9. Expression analyses showed that a gene homologous to GLABRA1 (GL1), a transcription factor implicated in trichome development in Arabidopsis thaliana, and the sequences 3'-flanking (downstream) of BrGL1, differed considerably between Mizuna (Kyo-mizore) and Mibuna (Kyo-nishiki). These results indicate that BrGL1 on LG A9 is one of the candidate genes responsible for the difference in trichome number between Mizuna and Mibuna. Detecting genes that are responsible for morphological variations allows us to better understand the breeding history of Mizuna and Mibuna.
The LG/J murine strain exhibits near-normal tendon biomechanical properties following a full-length central patellar tendon defect.[Pubmed:27552106]
Connect Tissue Res. 2016 Nov;57(6):496-506.
PURPOSE OF THE STUDY: Identifying biological success criteria is needed to improve therapies, and one strategy for identifying them is to analyze the RNA transcriptome for successful and unsuccessful models of tendon healing. We have characterized the MRL/MpJ murine strain and found improved mechanical outcomes following a central patellar tendon (PT) injury. In this study, we evaluate the healing of the LG/J murine strain, which comprises 75% of the MRL/MpJ background, to determine if the LG/J also exhibits improved biomechanical properties following injury and to determine differentially expressed transcription factors across the C57BL/6, MRL/MpJ and the LG/J strains during the early stages of healing. MATERIALS AND METHODS: A full-length, central PT defect was created in 16-20 week old MRL/MpJ, LG/J, and C57BL/6 murine strains. Mechanical properties were assessed at 2, 5, and 8 weeks post surgery. Transcriptomic expression was assessed at 3, 7, and 14 days following injury using a novel clustering software program to evaluate differential expression of transcription factors. RESULTS: Average LG/J structural properties improved to 96.7% and 97.2% of native LG/J PT stiffness and ultimate load by 8 weeks post surgery, respectively. We found the LG/J responded by increasing expression of transcription factors implicated in the inflammatory response and collagen fibril organization. CONCLUSIONS: The LG/J strain returns to normal structural properties by 8 weeks, with steadily increasing properties at each time point. Future work will characterize the cell populations responding to injury and investigate the role of the differentially expressed transcription factors during healing.
The "Phantom Effect" of the Rexinoid LG100754: structural and functional insights.[Pubmed:21152046]
PLoS One. 2010 Nov 30;5(11):e15119.
Retinoic acid receptors (RARs) and Retinoid X nuclear receptors (RXRs) are ligand-dependent transcriptional modulators that execute their biological action through the generation of functional heterodimers. RXR acts as an obligate dimer partner in many signalling pathways, gene regulation by rexinoids depending on the liganded state of the specific heterodimeric partner. To address the question of the effect of rexinoid antagonists on RAR/RXR function, we solved the crystal structure of the heterodimer formed by the ligand binding domain (LBD) of the RARalpha bound to its natural agonist ligand (all-trans retinoic acid, atRA) and RXRalpha bound to a rexinoid antagonist (LG100754). We observed that RARalpha exhibits the canonical agonist conformation and RXRalpha an antagonist one with the C-terminal H12 flipping out to the solvent. Examination of the protein-LG100754 interactions reveals that its propoxy group sterically prevents the H12 associating with the LBD, without affecting the dimerization or the active conformation of RAR. Although LG100754 has been reported to act as a 'phantom ligand' activating RAR in a cellular context, our structural data and biochemical assays demonstrate that LG100754 mediates its effect as a full RXR antagonist. Finally we show that the 'phantom ligand effect' of the LG100754 is due to a direct binding of the ligand to RAR that stabilizes coactivator interactions thus accounting for the observed transcriptional activation of RAR/RXR.
International Union of Pharmacology. LXIII. Retinoid X receptors.[Pubmed:17132853]
Pharmacol Rev. 2006 Dec;58(4):760-72.
The physiological effects of retinoic acids (RAs) are mediated by members of two families of nuclear receptors, the retinoic acid receptors (RARs) and the retinoid X receptors (RXRs), which are encoded by three distinct human genes, RXRalpha, RXRbeta, and RXRgamma. RARs bind both all-trans- and 9-cis-RA, whereas only the 9-cis-RA stereoisomer binds to RXRs. As RXR/RAR heterodimers, these receptors control the transcription of RA target genes through binding to RA-response elements. This review is focused on the structure, mode of action, ligands, expression, and pharmacology of RXRs. Given their role as common partners to many other members of the nuclear receptor superfamily, these receptors have been the subject of intense scrutiny. Moreover, and despite numerous studies since their initial discovery, RXRs remain enigmatic nuclear receptors, and there is still no consensus regarding their role. Indeed, multiple questions about the actual biological role of RXRs and the existence of an endogenous ligand have still to be answered.
The antidiabetic agent LG100754 sensitizes cells to low concentrations of peroxisome proliferator-activated receptor gamma ligands.[Pubmed:11877384]
J Biol Chem. 2002 Apr 12;277(15):12503-6.
Insulin resistance and non-insulin-dependent diabetes mellitus are major causes of morbidity and mortality in industrialized nations. Despite the alarming rise in the prevalence of this disorder, the initial molecular events that promote insulin resistance remain unclear. The data presented here demonstrate that LG100754, an antidiabetic RXR ligand, defines a novel type of nuclear receptor agonist. Surprisingly, LG100754 has minimal intrinsic transcriptional activity, instead it enhances the potency of proliferator-activated receptor (PPAR) gamma-retinoid X receptor heterodimers for PPARgamma ligands. The ability of LG100754 to both increase PPARgamma sensitivity and relieve insulin resistance implies that a deficiency in endogenous PPARgamma ligands may represent an early step in the development of insulin resistance.
The rexinoid LG100754 is a novel RXR:PPARgamma agonist and decreases glucose levels in vivo.[Pubmed:11463859]
Mol Endocrinol. 2001 Aug;15(8):1360-9.
The RXR serves as a heterodimer partner for the PPARgamma and the dimer is a molecular target for insulin sensitizers such as the thiazolidinediones. Ligands for either receptor can activate PPAR-dependent pathways via PPAR response elements. Unlike PPARgamma agonists, however, RXR agonists like LG100268 are promiscuous and activate multiple RXR heterodimers. Here, we demonstrate that LG100754, a RXR:RXR antagonist and RXR:PPARalpha agonist, also functions as a RXR:PPARgamma agonist. It does not activate other LG100268 responsive heterodimers like RXR:liver X receptoralpha, RXR:liver X receptorbeta, RXR:bile acid receptor/farnesoid X receptor and RXR:nerve growth factor induced gene B. This unique RXR ligand triggers cellular RXR:PPARgamma-dependent pathways including adipocyte differentiation and inhibition of TNFalpha-mediated hypophosphorylation of the insulin receptor, but does not activate key farnesoid X receptor and liver X receptor target genes. Also, LG100754 treatment of db/db animals leads to an improvement in insulin resistance in vivo. Interestingly, activation of RXR:PPARgamma by LG100268 and LG100754 occurs through different mechanisms. Therefore, LG100754 represents a novel class of insulin sensitizers that functions through RXR but exhibits greater heterodimer selectivity compared with LG100268. These results establish an approach to the design of novel RXR-based insulin sensitizers with greater specificity.


