NeuchromeninCAS# 180964-26-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 180964-26-5 | SDF | Download SDF |
| PubChem ID | 10729197 | Appearance | Powder |
| Formula | C13H12O5 | M.Wt | 248.23 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
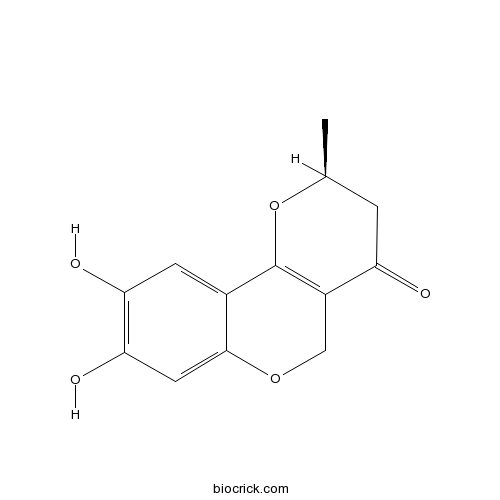
| Chemical Name | (2S)-8,9-dihydroxy-2-methyl-3,5-dihydro-2H-pyrano[3,2-c]chromen-4-one | ||
| SMILES | CC1CC(=O)C2=C(O1)C3=CC(=C(C=C3OC2)O)O | ||
| Standard InChIKey | SGTSJAOFFFAYJH-LURJTMIESA-N | ||
| Standard InChI | InChI=1S/C13H12O5/c1-6-2-9(14)8-5-17-12-4-11(16)10(15)3-7(12)13(8)18-6/h3-4,6,15-16H,2,5H2,1H3/t6-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. (-)-Neuchromenin is an inducer of neurite outgrowth of PC12 cells at concentration of 2.5-10 ug/ml. |

Neuchromenin Dilution Calculator

Neuchromenin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0285 mL | 20.1426 mL | 40.2852 mL | 80.5704 mL | 100.713 mL |
| 5 mM | 0.8057 mL | 4.0285 mL | 8.057 mL | 16.1141 mL | 20.1426 mL |
| 10 mM | 0.4029 mL | 2.0143 mL | 4.0285 mL | 8.057 mL | 10.0713 mL |
| 50 mM | 0.0806 mL | 0.4029 mL | 0.8057 mL | 1.6114 mL | 2.0143 mL |
| 100 mM | 0.0403 mL | 0.2014 mL | 0.4029 mL | 0.8057 mL | 1.0071 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Spiramilactone B
Catalog No.:BCN1141
CAS No.:180961-65-3
- Jaceosidin
Catalog No.:BCN2529
CAS No.:18085-97-7
- 4-O-Caffeoylshikimic acid
Catalog No.:BCN7931
CAS No.:180842-65-3
- CHMFL-ABL-053
Catalog No.:BCC3988
CAS No.:1808287-83-3
- LG 100754
Catalog No.:BCC7786
CAS No.:180713-37-5
- Polypodine B
Catalog No.:BCN8117
CAS No.:18069-14-2
- 1,4-Bis(5-phenyl-2-oxazolyl)benzene
Catalog No.:BCC8424
CAS No.:1806-34-4
- 2,2'-Biphenol
Catalog No.:BCC8488
CAS No.:1806-29-7
- Peiminine
Catalog No.:BCN1095
CAS No.:18059-10-4
- Gentioflavin
Catalog No.:BCN3619
CAS No.:18058-50-9
- Fmoc-D-Asn(Trt)-OH
Catalog No.:BCC3084
CAS No.:180570-71-2
- Solifenacin hydrochloride
Catalog No.:BCC5193
CAS No.:180468-39-7
- FTI 277 HCl
Catalog No.:BCC6395
CAS No.:180977-34-8
- 3-O-Caffeoylshikimic acid
Catalog No.:BCN7930
CAS No.:180981-12-8
- Curcumaromin A
Catalog No.:BCN7417
CAS No.:1810034-38-8
- Curcumaromin B
Catalog No.:BCN7419
CAS No.:1810034-39-9
- Curcumaromin C
Catalog No.:BCN7418
CAS No.:1810034-40-2
- Corymbosin
Catalog No.:BCN6812
CAS No.:18103-41-8
- 5,7-Dihydroxy-3',4',5'-trimethoxyflavone
Catalog No.:BCN6807
CAS No.:18103-42-9
- Proflavine Hemisulfate
Catalog No.:BCC4707
CAS No.:1811-28-5
- 5-Hydroxy-4-methoxycanthin-6-one
Catalog No.:BCN1142
CAS No.:18110-86-6
- 4,5-Dimethoxycanthin-6-one
Catalog No.:BCN1143
CAS No.:18110-87-7
- Co 102862
Catalog No.:BCC7439
CAS No.:181144-66-1
- Almotriptan Malate
Catalog No.:BCC5045
CAS No.:181183-52-8
84(P-41) Determination of the Absolute Configuration of Microbial Metabolites, (-)-Neuchromenin, (-)-Nocardione A and (-)-Nocardione B by Their Synthesis
Natural Organic Compounds Symposium keynote speech (43), 2001: 497-502.
In 1996 (-)-Neuchromenin (1) was isolated from the culture broth of Eupenicillium javanicum var. meloforme PF1181 as an inducer of neurite outgrowth of PC12 cells at concentration of 2.5-10 μg/ml. Its structure was deduced as 1 by extensive spectral analysis, although its absolute configuration remained unknown. In order to settle that stereochemical problem, we undertook the synthesis of both the enantiomers of Neuchromenin using both the enantiomers of known aldehyde (11). By synthesizing both (R) and (S) enantiomers of Neuchromenin, we found (S) enantiomer to be identical with the naturally occurring (-)-Neuchromenin. In 2000 two new furano-o-naphthoquinones, (-)-nocardione B (2) and (-)-nocardione A (3) were isolated as new tyrosine phosphatase inhibitors with moderate antifungal and cytotoxic activities. Due to the scarcity of the materials, their absolute configuration remained unknown. In order to solve this problem, we undertook a synthesis of optically active nocardiones with known absolute configuration. (R)-(+)-Nocardione B (2) and (S)-(-)-nocardione A (3) were synthesized by starting from the commercially available enantiomers of propylene oxide and 5-benzyloxy (or methoxy)-1-tetralone. As a result, the absolute configuration of the naturally occurring (-)-nocardione A and (-)-nocardione B was established as S.


