Solifenacin hydrochlorideCAS# 180468-39-7 |
- Resminostat hydrochloride
Catalog No.:BCC1888
CAS No.:1187075-34-8
- RG2833
Catalog No.:BCC1893
CAS No.:1215493-56-3
- Daminozide
Catalog No.:BCC1514
CAS No.:1596-84-5
- Tasquinimod
Catalog No.:BCC1987
CAS No.:254964-60-8
- CHAPS
Catalog No.:BCC1476
CAS No.:75621-03-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 180468-39-7 | SDF | Download SDF |
| PubChem ID | 154058 | Appearance | Powder |
| Formula | C23H27ClN2O2 | M.Wt | 398.93 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | YM905 hydrochloride | ||
| Solubility | DMSO : 50 mg/mL (125.34 mM; Need ultrasonic) | ||
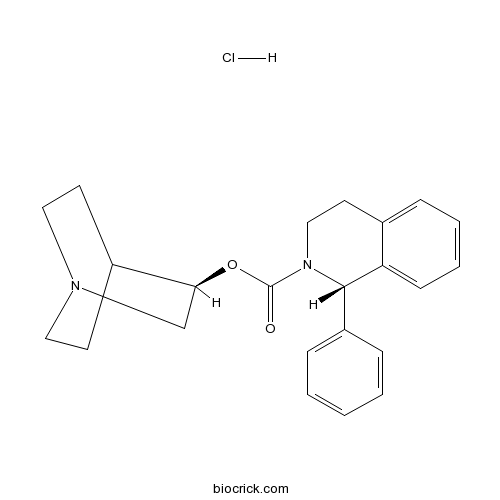
| Chemical Name | [(3R)-1-azabicyclo[2.2.2]octan-3-yl] (1S)-1-phenyl-3,4-dihydro-1H-isoquinoline-2-carboxylate;hydrochloride | ||
| SMILES | C1CN2CCC1C(C2)OC(=O)N3CCC4=CC=CC=C4C3C5=CC=CC=C5.Cl | ||
| Standard InChIKey | YAUBKMSXTZQZEB-VROPFNGYSA-N | ||
| Standard InChI | InChI=1S/C23H26N2O2.ClH/c26-23(27-21-16-24-13-10-18(21)11-14-24)25-15-12-17-6-4-5-9-20(17)22(25)19-7-2-1-3-8-19;/h1-9,18,21-22H,10-16H2;1H/t21-,22-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Solifenacin Hcl(YM905 Hcl; Vesicare Hcl) is a muscarinic receptor antagonist.
IC50 value:
Target: muscarinic receptor
Solifenacin Hcl (YM905; Vesicare) is a prescription medication used to treat certain bladder problems. References: | |||||

Solifenacin hydrochloride Dilution Calculator

Solifenacin hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5067 mL | 12.5335 mL | 25.0671 mL | 50.1341 mL | 62.6676 mL |
| 5 mM | 0.5013 mL | 2.5067 mL | 5.0134 mL | 10.0268 mL | 12.5335 mL |
| 10 mM | 0.2507 mL | 1.2534 mL | 2.5067 mL | 5.0134 mL | 6.2668 mL |
| 50 mM | 0.0501 mL | 0.2507 mL | 0.5013 mL | 1.0027 mL | 1.2534 mL |
| 100 mM | 0.0251 mL | 0.1253 mL | 0.2507 mL | 0.5013 mL | 0.6267 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Solifenacin Hcl(YM905 Hcl; Vesicare Hcl) is a muscarinic receptor antagonist.
- Perillaldehyde
Catalog No.:BCN8294
CAS No.:18031-40-8
- Cyclo(Leu-Ala)
Catalog No.:BCN2428
CAS No.:1803-60-7
- NCT-501
Catalog No.:BCC6539
CAS No.:1802088-50-1
- Phytolaccagenin
Catalog No.:BCN1140
CAS No.:1802-12-6
- Ganoderlactone D
Catalog No.:BCN7849
CAS No.:1801934-15-5
- Anthracophyllone
Catalog No.:BCN7606
CAS No.:1801750-22-0
- Megastigm-7-ene-3,4,6,9-tetrol
Catalog No.:BCN6511
CAS No.:180164-14-1
- Quercetin 3-O-glucoside-7-O-rhamnoside
Catalog No.:BCN1520
CAS No.:18016-58-5
- Bupivacaine HCl
Catalog No.:BCC4406
CAS No.:18010-40-7
- SB 224289 hydrochloride
Catalog No.:BCC6976
CAS No.:180084-26-8
- Voafinidine
Catalog No.:BCN6738
CAS No.:180059-77-2
- SDZ NKT 343
Catalog No.:BCC7349
CAS No.:180046-99-5
- Fmoc-D-Asn(Trt)-OH
Catalog No.:BCC3084
CAS No.:180570-71-2
- Gentioflavin
Catalog No.:BCN3619
CAS No.:18058-50-9
- Peiminine
Catalog No.:BCN1095
CAS No.:18059-10-4
- 2,2'-Biphenol
Catalog No.:BCC8488
CAS No.:1806-29-7
- 1,4-Bis(5-phenyl-2-oxazolyl)benzene
Catalog No.:BCC8424
CAS No.:1806-34-4
- Polypodine B
Catalog No.:BCN8117
CAS No.:18069-14-2
- LG 100754
Catalog No.:BCC7786
CAS No.:180713-37-5
- CHMFL-ABL-053
Catalog No.:BCC3988
CAS No.:1808287-83-3
- 4-O-Caffeoylshikimic acid
Catalog No.:BCN7931
CAS No.:180842-65-3
- Jaceosidin
Catalog No.:BCN2529
CAS No.:18085-97-7
- Spiramilactone B
Catalog No.:BCN1141
CAS No.:180961-65-3
- Neuchromenin
Catalog No.:BCN7449
CAS No.:180964-26-5
Efficacy and safety of tamsulosin hydrochloride 0.2 mg and combination of tamsulosin hydrochloride 0.2 mg plus solifenacin succinate 5 mg after transurethral resection of the prostate: a prospective, randomized controlled trial.[Pubmed:27698559]
Clin Interv Aging. 2016 Sep 19;11:1301-1307.
OBJECTIVE: The objective of this study was to evaluate the safety and efficacy of tamsulosin hydrochloride 0.2 mg (TAM) and its combination with solifenacin succinate 5 mg (SOL) after transurethral resection of the prostate (TURP). PATIENTS AND METHODS: The patients were randomized into three groups: TURP (group 1), TURP plus TAM (group 2), and TURP plus TAM + SOL (group 3). Patients in group 2 and group 3 received medication for 4 weeks. The primary efficacy end points were the mean change in total International Prostate Symptom Score (IPSS) and IPSS subscores. The secondary end points included quality-of-life score, Overactive Bladder Symptom Score, and short-form voiding and storage score of International Continence Society. RESULTS: In total, 37 men (31.8%) in group 1, 37 men (31.8%) in group 2, and 42 men (36.2%) in group 3 completed the study. In total IPSS, no significant improvement was seen from baseline to the end of treatment in groups 2 and 3 compared with group 1. However, in group 2, the decrement in the IPSS storage score was smaller than group 1 (P=0.02), and in group 3, the decrement in the IPSS voiding score was smaller than group 1 (P=0.05). In groups 2 and 3 compared with group 1, improvements in the quality of life score, total score of Overactive Bladder Symptom Score, and short-form voiding score and storage score of International Continence Society were not statistically significant. CONCLUSION: Treatment with TAM and combination of TAM and SOL did not have significant additional benefits for lower urinary tract symptoms during the early recovery period after TURP.


