Picrasma quassioides
Picrasma quassioides
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Picrasma quassioides
- Cat.No. Product Name CAS Number COA
-
BCN1236
Maackiain19908-48-6
Instructions

-
BCN2633
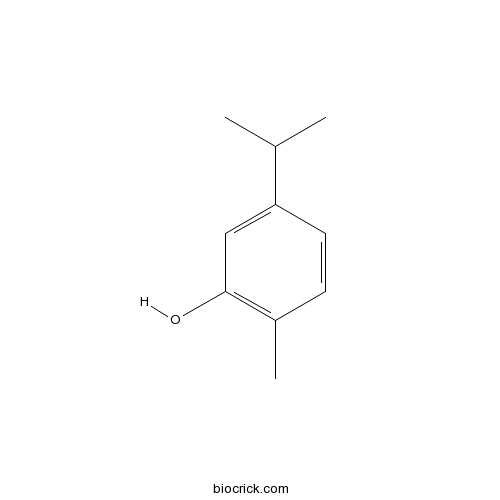
5-Isopropyl-2-methylphenol499-75-2
Instructions
-
BCN5649
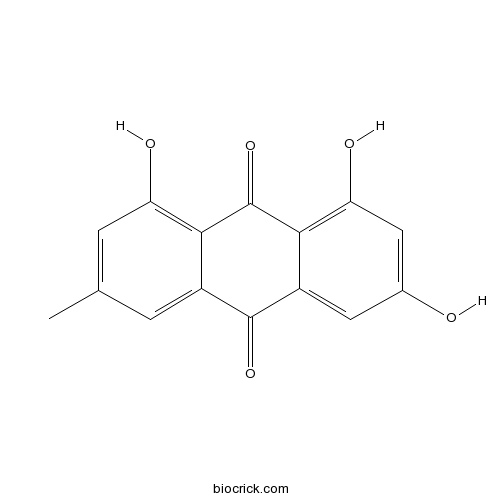
Emodin518-82-1
Instructions
-
BCN4237
Trifolirhizin6807-83-6
Instructions

Bioactivity-guided isolation of β-Carboline alkaloids with potential anti-hepatoma effect from Picrasma quassioides (D. Don) Benn.[Pubmed: 30114469]
β-Carboline alkaloids in Picrasma quassioides (D. Don) Benn. have been proven to possess inhibitory activity against various cancer cells. However, their effect on hepatocellular carcinoma and structure-activity relationships (SAR) have not been systematically reported. In this work, bioactivity-directed fractionation of P. quassioides led to the separation of active fraction A2-2. A total of 39 β-carbolines, including 4 new ones (1-4), were obtained from the active fraction. Moreover, all the isolated compounds were identified in the active fraction A2-2 by LC-MS. The cytotoxicity on HepG2 and Hep3B cells of all compounds was screened by MTT assay, and the SAR were established. The SAR were also supported by the apoptosis ratio of HepG2 cells using flow cytometry analysis after treatment with potential compounds 1, 2, 9, 10, 12, 29, 36 and 38. It suggested that these active compounds caused death of hepatoma cells through apoptosis induction. In addition, further study revealed that compounds 12, 29, 36 significantly activated caspase-3 in HepG2 cells.
Preparation and purification of canthinone and β-carboline alkaloids from Picrasma quassioides based on bioautography and mass-spectrometry-directed autopurification system.[Pubmed: 29797546]
None
Two new β-carboline alkaloids from the stems of Picrasma quassioides.[Pubmed: 29725985]
Two new β-carboline alkaloids, 1-acetyl-4-methoxy-8-hydroxy-β-carboline (1) and 1-acetyl-4,8-dimethoxy-β-carboline (2), together with 10 known compounds; seven β-carboline alkaloids (3-9), two canthin-6-one alkaloids (10 and 11), and one quassinoid (12) were isolated from the stems of Picrasma quassioides. The structure of the new compounds 1 and 2 were determined by spectroscopic analyses including 1D- and 2D-NMR and HRMS interpretation. All the isolates (1-12) were evaluated for their cytotoxicity against human ovarian carcinoma A2780 and SKOV3 cell lines using MTT assays. Of the isolates, compounds 5-7 exhibited the most potent cytotoxicity on both A2780 and SKOV3 cell lines in vitro.
A new ionone derivative from the leaves of Picrasma quassioides.[Pubmed: 29717884]
Nigakialcohol A (1), as unusual cyclization ionone derivative, together with eight known ones (2-9), were isolated from the leaves of Picrasma quassioides (D. Don) Benn (Simaroubaceae). Their structures were elucidated by extensive spectroscopic analyses and comparison with literature data. Compound 2 showed a weak inhibitory effect on NO production at non-cytotoxic concentration (100 μM) with inhibitory rate of 59%, and thus it should be regarded as potential anti-inflammatory agents.
Enantiomeric neolignans from Picrasma quassioides exhibit distinctive cytotoxicity on hepatic carcinoma cells through ROS generation and apoptosis induction.[Pubmed: 29567344]
None
In vivo toxic effects of 4-methoxy-5-hydroxy-canthin-6-one in zebrafish embryos via copper dyshomeostasis and oxidative stress.[Pubmed: 29208543]
Dysfunction of copper homeostasis can lead to a host of disorders, which might be toxic sometimes. 4-Methoxy-5-hydroxy-canthin-6-one (CAN) is one of the major constituents from Picrasma quassioides and responsible for its therapeutic effects. In this work, we evaluated the toxic effect of CAN (7.5μM) on zebrafish embryos. CAN treatment decreased survival, delayed hatching time and induced malformations (loss of pigmentation, pericardial edema, as well as hematologic and neurologic abnormalities). Besides, exogenous copper supplementation rescued the pigmentation and cardiovascular defects in CAN-treated embryos. Further spectroscopic studies revealed a copper-chelating activity of CAN. Then its regulation on the expressions of copper homeostasis related genes also be analyzed. In addition, CAN lowered the total activity of SOD, elevated the ROS production and altered the oxidative related genes transcriptions, which led to oxidative stress. In conclusion, we demonstrated that CAN (7.5μM) might exert its toxic effects in zebrafish embryos by causing copper dyshomeostasis and oxidative stress. It will give insight into the risk assessment and prevention of CAN-mediated toxicity.
A self-feedback network based on liquid chromatography-quadrupole-time of flight mass spectrometry for system identification of β-carboline alkaloids in Picrasma quassioides.[Pubmed: 29062115]
Profiling chemical components in herbs by mass spectrometry is a challenging work because of the lack of standard compounds, especially for position isomers. This paper provides a strategy based on a self-feedback network of mass spectra (MS) data to identify chemical constituents in herbs by liquid chromatography-quadrupole-time of flight mass spectrometry without compound standards. Components sharing same skeleton were screened and all ions were classified into a database. All candidates were connected by the selected bridging ions to establish a primary MS network. Benefited from such a network, it is feasible to characterize sequentially the structures of all diagnostic ions and candidates once single component has been de novo identified. Taking Picrasma quassioides as an example, the primary network of β-carbolines was established with 65 ions (selected from 76 β-carbolines), each of which appeared at least in four compounds. Once an alkaloid has been identified, its logical ions could feedback into primary network to build pathways with other unknown compounds. Moreover, the position of the substituent groups could be deduced through the secondary metabolic pathways of alkaloids (plant secondary metabolism). The network therefore can be utilized for identification of unknown compounds and even their position isomers.


