Rhaphidophora decursiva
Rhaphidophora decursiva
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Rhaphidophora decursiva
- Cat.No. Product Name CAS Number COA
-
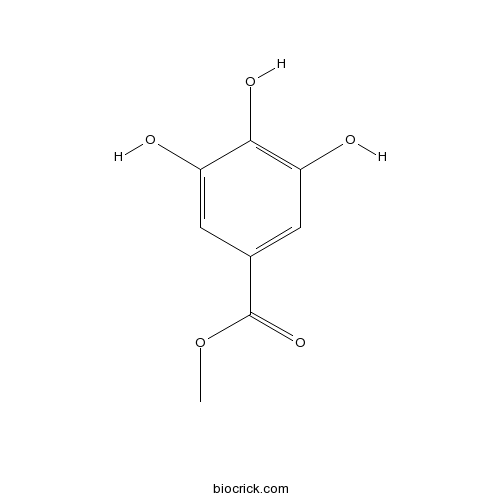
BCN3823
Methyl gallate99-24-1
Instructions
-
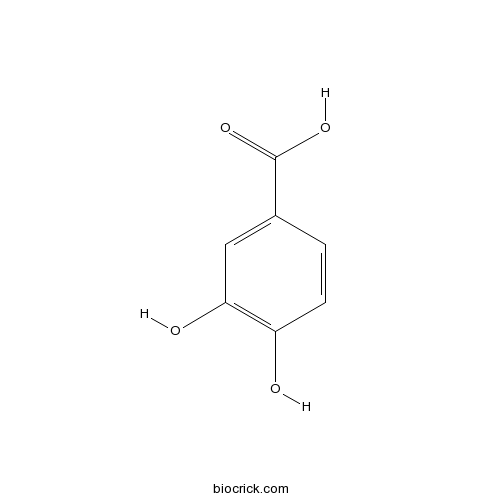
BCN4537
3,4-Dihydroxybenzoic acid99-50-3
Instructions
Three new species of Piricaudiopsis from southern China.[Pubmed: 19537214]
Piricaudiopsis punicae sp. nov., P. rosae sp. nov. and P. rhaphidophorae sp. nov., occurring respectively on dead branches of Punica granatum, Rosa chinensis and Rhaphidophora decursiva were collected from tropical forest in southern China. They differ from the three previously described Piricaudiopsis species in conidial morphology and proliferation of the conidiogenous cell. The presence or absence of percurrent proliferation of the conidiogenous cells and conidial appendages and the height of conidia are recognized as important characters in the delimitation of species of Piricaudiopsis. A key to Piricaudiopsis species is provided.
[Fog water absorption by the leaves of epiphytes and non - epiphytes in Xishuangbanna].[Pubmed: 16964926]
Xishuangbanna is located at the northern margin of tropics. Its climate is different from that of typical tropics, but the rainforest there is not very different from that of the typical tropics in Southeast Asia. The main problems in Xishuangbanna are seasonal drought and low temperature. Fog may contribute to the development of rainforest here, but related studies are few. This study is aimed to know whether the leaves of epiphytes and non - epiphytes in Xishuangbanna can directly absorb fog water and contribute to their water status recovery, and whether epiphytes are more competent than non - epiphytes in their leaf fog water absorption. The study was conducted in dry season, and four species of epiphytes and six species of non - epiphytes were investigated. The effect of fog was imitated by spraying leaves with distilled water. For epiphytes and non - epiphytes, their leaf water potential (phi), relative water content (RWC), and amount of absorbed water increased gradually with the time of spraying, but the phi of epiphytes increased more quickly than that of non - epiphytes. The leaves of epiphytes Bolbitis scandens and Rhaphidophora decursiva could absorb fog water more quickly, and increase their RWC more greatly than those of non - epiphytes, indicating that these epiphytes were more competent than non - epiphytes in their leaf fog water absorption. The fog water absorption capacity of the leaves in epiphytic orchid Coelogyne occultata and Staurochilus dawsonianus was lower than that in Amischotolype hispida and Mananthus patentflora, but higher than that in other four non - epiphytes. The phi of epiphytes at early evening when no fog was formed was significantly lower than that at early morning, suggesting that fog water was absorbed by epiphytes at night to improve their leaf water status. Non - epiphytes did not need to absorb fog water directly through leaves, and they could recover their leaf water status through absorbing soil water by root system. Epiphytes except C. occultata had a much more leaf biomass than non - epiphytes, which was also beneficial to their leaf fog water absorption. Because there was abundant fog in dry season in Xishuangbanna, the phi of test ten species was higher than -0.8 MPa, indicating that water stress was not serious in dry season.
Antimalarial agents from plants. III. Trichothecenes from Ficus fistulosa and Rhaphidophora decursiva.[Pubmed: 12494335]
Bioassay-directed fractionation of an extract prepared from the dried leaves and stem barks of Ficus fistulosa Reinw. ex Blume (Moraceae) led to the isolation of verrucarin L acetate (1), together with 3alpha-hydroxyisohop-22(29)-en-24-oic acid, 3beta-gluco-sitosterol, 3,4-dihydro-6,7-dimethoxyisocarbostyril, 3,4,5-trimethoxybenzyl alcohol, alpha-methyl-3,4,5-trimethoxybenzyl alcohol, indole-3-carboxaldehyde, palmanine, and aurantiamide acetate. Roridin E (2) was identified in a subfraction from the dried leaves and stems of Rhaphidophora decursiva Schott (Araceae). Verrucarin L acetate and roridin E were characterized as macrocyclic trichothecene sesquiterpenoids and found to inhibit the growth of Plasmodium falciparum with IC 50 values below 1 ng/ml.
Antimalarial compounds from Rhaphidophora decursiva.[Pubmed: 11421741]
Bioassay-directed fractionation led to the isolation of 14 compounds, six of which possess antimalarial activity, from the dried leaves and stems of Rhaphidophora decursiva. Polysyphorin (1) and rhaphidecurperoxin (6) showed strong activities against Plasmodium falciparum. Rhaphidecursinol A (2), rhaphidecursinol B (3), grandisin (4), and epigrandisin (5) were less active against the same organism. Among the isolates, rhaphidecursinol A (2) and rhaphidecursinol B (3) were determined to be new neolignans, and rhaphidecurperoxin (6) is a new benzoperoxide. Known compounds isolated include polysyphorin (1), grandisin (4), epigrandisin (5), (+)-medioresinol, (-)-pinoresinol, (-)-syringaresinol, (+)-glaberide I, (+)-dehydrovomifoliol, (-)-liliolide, (-)-hydroxydihydrobovolide, and N-butylbenzamide, of which compound 1 appears worthy of further evaluation as an antimalarial agent. Structure elucidation and identification were accomplished by spectroscopic means including 1D and 2D NMR analyses.


