Rhododendron fortunei
Rhododendron fortunei
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Rhododendron fortunei
- Cat.No. Product Name CAS Number COA
-
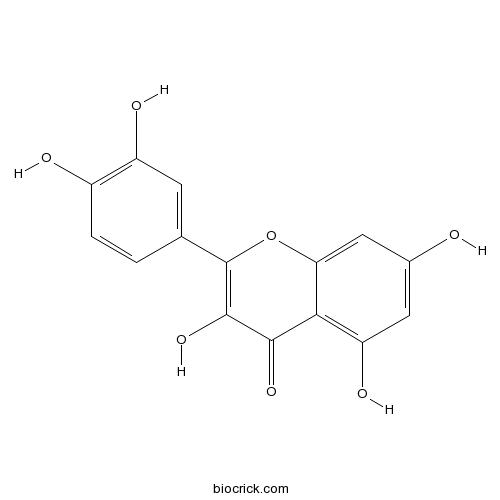
BCN6049
Quercetin117-39-5
Instructions
-
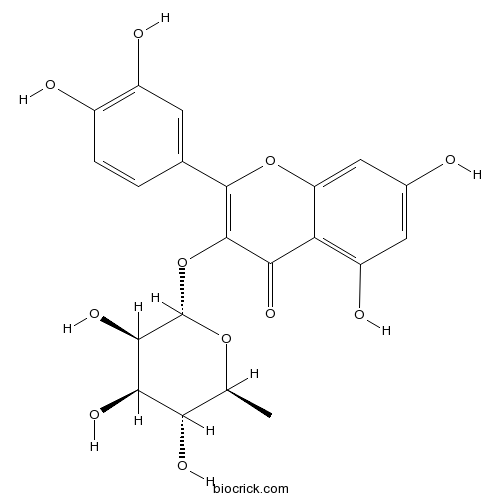
BCN5665
Quercitrin522-12-3
Instructions
-
BCN5692
Myricetin529-44-2
Instructions

-
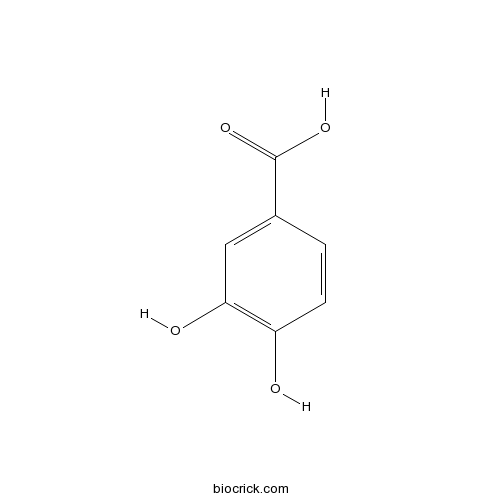
BCN4537
3,4-Dihydroxybenzoic acid99-50-3
Instructions
A New Oidiodendron maius Strain Isolated from Rhododendron fortunei and its Effects on Nitrogen Uptake and Plant Growth.[Pubmed: 27602030]
A new mycorrhizal fungal strain was isolated from hair roots of Rhododendron fortunei Lindl. grown in Huading Forest Park, Zhejiang Province, China. Morphological characterization and internal transcribed spacer rDNA analysis suggested that it belongs to Oidiodendron maius Barron, and we designated it as strain Om19. Methods for culturing Om19 were established, and the ability of Om19 to form mycorrhizae on R. fortunei was evaluated in a peat-based substrate. Microscopic observations showed hyaline hyphae on the surface of hair roots and crowded hyphal complexes (hyphal coils) inside root cortical cells of R. fortunei after inoculation, indicating that the roots were well colonized by Om19. In a second experiment, fresh and dry weight of R. fortunei 2 months after Om19 inoculation were greater than uninoculated plants, and the total nitrogen absorbed by plants inoculated with Om19 was greater than the uninoculated controls. qRT-PCR analysis of five genes related to N uptake and metabolism (two nitrate transporters, an ammonium transporter, glutamine synthetase, and glutamate synthase) showed that these genes were highly upregulated with twofold to ninefold greater expression in plants inoculated with Om19 compared to uninoculated plants. In the third experiment, Om19 was inoculated into the peat-based substrate for growing Formosa azalea (Rhododendron indica 'Formosa'). 'Formosa' azalea plants grown in the inoculated substrate had larger canopies and root systems compared to uninoculated plants. Our results show that Om19 could be an important microbial tool for improving production of Rhododendron plants.
6,8-Di-C-methyl-flavonoids with neuroprotective activities from Rhododendron fortunei.[Pubmed: 27345941]
Six 6,8-di-C-methyl-flavonoids, (2R,3R)-6,8-di-C-methyl-5,7,4'-trihydroxyflavanonol 7-O-β-d-gluco-pyranoside (1), (2R,3R)-6,8-di-C-methyl-5,7,4'-trihydroxyflavanonol 7-O-β-d-xylopyranosyl(1→6)-β-d-glucopyranoside (2), 6,8-di-C-methylkaempferol 7-O-β-d-glucopyranoside (3), (2R)-farrerol (4a), (2R/2S)-farrerol 7-O-β-d-glucopyranoside (5), and (2R/2S)-farrerol 7-O-β-d-xylopyranosyl(1→6)-β-d-glucopyranoside (6), and four known analogues, farrerol (4b), (2R,3R)-6,8-di-C-methyldihydrokae-mpferol (7), 6,8-di-C-methylkaempferol 7-O-β-d-glucopyranoside (8), and 6,8-di-C-methylkaempferol (9), were isolated from the twigs and leaves of Rhododendron fortunei. The structures of compounds 1-9 were determined by spectroscopic analyses (HRESIMS, 1D and 2D NMR, and CD) and chemical methods. Compounds 1-9 were evaluated for their neuroprotective effects on the human neuroblastoma SH-SY5Y cells apoptosis induced by hydrogen peroxide (H2O2) and amyloid β peptide (Aβ), respectively. Compounds 1-3 and 5-9 exhibited significant neuroprotective effects against H2O2-induced SH-SY5Y cell apoptosis, and compound 8 exhibited the strongest activity with a improvement of cell viability by about 30% at the concentration of 10μM. Compounds 1-9 showed significant neuroprotective effects against Aβ-induced SH-SY5Y cell apoptosis.
Identification and characterization of proanthocyanidins of 16 members of the Rhododendron genus (Ericaceae) by tandem LC-MS.[Pubmed: 22689627]
The proanthocyanidins of the leaves of 16 taxa of the Rhododendron genus (Ericaceae) [Rhododendron 'Catawbiense Grandiflorum', Rhododendron 'Cunningham's White', Rhododendron smirnowii Trautv., Rhododendron calophytum Franch., Rhododendron dichroanthum ssp. scyphocalyx (Balf. f. & Forrest ) Cowan, Rhododendron micranthum Turcz., Rhododendron praevernum Hutch., Rhododendron ungernii Trautv., Rhododendron kaempferi Planch., Rhododendron degronianum ssp. heptamerum var. hondoense (Nakai ) H. Hara, Rhododendron fortunei Lindl., Rhododendron ponticum L., Rhododendron galactinum Balf. f. ex Tagg., Rhododendron oreotrephes W. W. Sm., Rhododendron brachycarpum ssp. brachycarpum D. Don ex G. Don, and Rhododendron insigne Hemsl. & E. H. Wilson ] were investigated qualitatively by liquid chromatography-mass spectrometry in series. Twenty-nine dimeric proanthocyanidins based on (epi)catechin and (epi)gallocatechin were detected and characterized on the basis of their unique fragmentation pattern in the negative ion mode tandem mass spectrometry spectra. All of them were extracted for the first time from these sources, and ten of them were not reported previously in nature. The position of the galloyl residue was assigned on the basis of the retro-Diels-Alder fragmentation and the dehydrated retro-Diels-Alder fragmentation; it resulted from the loss of gallic acid as a neutral loss in the negative ion mode. Furthermore, four caffeoylquinic acids, six p-coumaroylquinic acids, epigallocatechin, gallocatechin, catechin, epicatechin, epigallocatechin gallate, catechin gallate, epicatechin gallate, gallocatechin gallate, two quercetin-O-hexosides, quercetin-O-galloyl-hexoside, quercetin-O-pentoside, quercetin-O-rhamnoside, quercetin-O-pentoside-O-hexoside, quercetin-O-rhamnoside-O-hexoside, quercetin-O-feruloyl-hexoside, quercetin-O-(p-hydroxy)benzoyl-hexoside, taxifolin-O-pentoside, myricetin-O-rhamnoside, two myricetin-O-pentosides, three myricetin-O-hexosides, and two myricetin-O-galloyl-hexosides were detected and shown to possess characteristic tandem mass spectrometry spectra and were tentatively assigned on the basis of their retention time.
Diversity of root-associated fungal endophytes in Rhododendron fortunei in subtropical forests of China.[Pubmed: 19396474]
To investigate the diversity of root endophytes in Rhododendron fortunei, fungal strains were isolated from the hair roots of plants from four habitats in subtropical forests of China. In total, 220 slow-growing fungal isolates were isolated from the hair roots of R. fortunei. The isolates were initially grouped into 17 types based on the results of internal transcribed spacer-restriction fragment length polymorphism (ITS-RFLP) analysis. ITS sequences were obtained for representative isolates from each RFLP type and compared phylogenetically with known sequences of ericoid mycorrhizal endophytes and selected ascomycetes or basidiomycetes. Based on phylogenetic analysis of the ITS sequences in GenBank, 15 RFLP types were confirmed as ascomycetes, and two as basidiomycetes; nine of these were shown to be ericoid mycorrhizal endophytes in experimental cultures. The only common endophytes of R. fortunei were identified as Oidiodendron maius at four sites, although the isolation frequency (3-65%) differed sharply according to habitat. Phialocephala fortinii strains were isolated most abundantly from two habitats which related to the more acidic soil and pine mixed forests. A number of less common mycorrhizal RFLP types were isolated from R. fortunei at three, two, or one of the sites. Most of these appeared to have strong affinities for some unidentified root endophytes from Ericaceae hosts in Australian forests. We concluded that the endophyte population isolated from R. fortunei is composed mainly of ascomycete, as well as a few basidiomycete strains. In addition, one basidiomycete strain was confirmed as a putative ericoid mycorrhizal fungus.


