Sophora flavescens
Sophora flavescens
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Sophora flavescens
- Cat.No. Product Name CAS Number COA
-
BCN1103
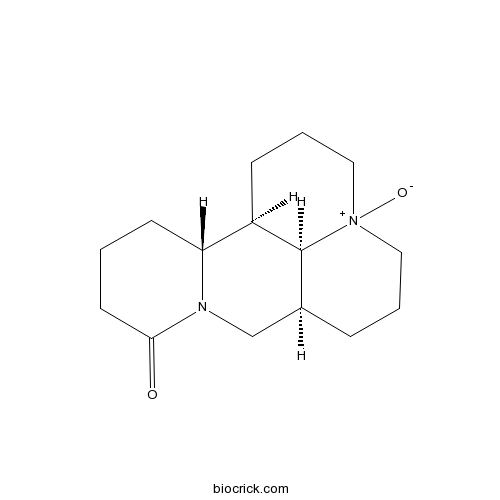
Oxymatrine16837-52-8
Instructions
-
BCN1236
Maackiain19908-48-6
Instructions

-
BCN1503
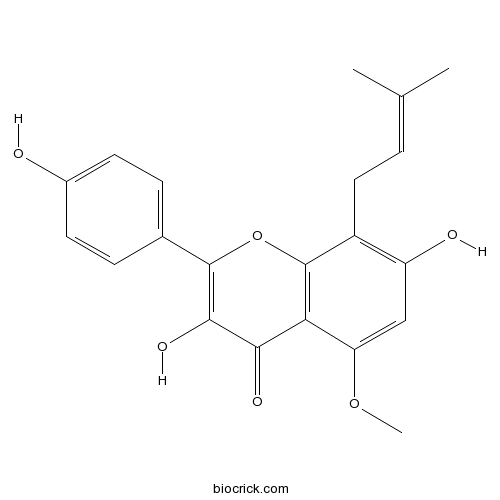
3,7,4'-Trihydroxy-5-methoxy-8-prenylflavanone204935-85-3
Instructions

-
BCN2891
Sophoflavescenol216450-65-6
Instructions
-
BCN5156
Oxysophocarpine26904-64-3
Instructions

-
BCN2986
2'-Methoxykurarinone270249-38-2
Instructions

-
BCN2985
Kurarinone34981-26-5
Instructions

-
BCN5351
Anhydroicaritin38226-86-7
Instructions

-
BCN6270
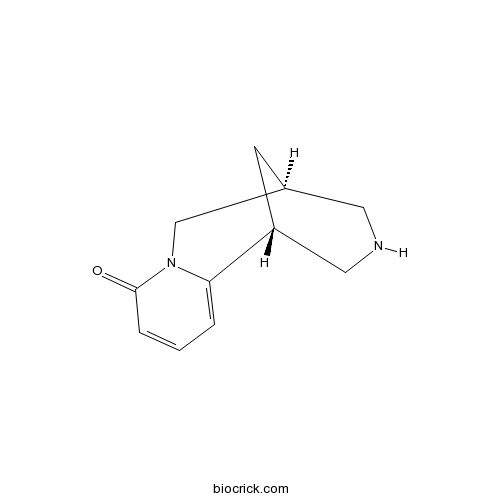
Cytisine485-35-8
Instructions
-
BCN5650
Matrine519-02-8
Instructions
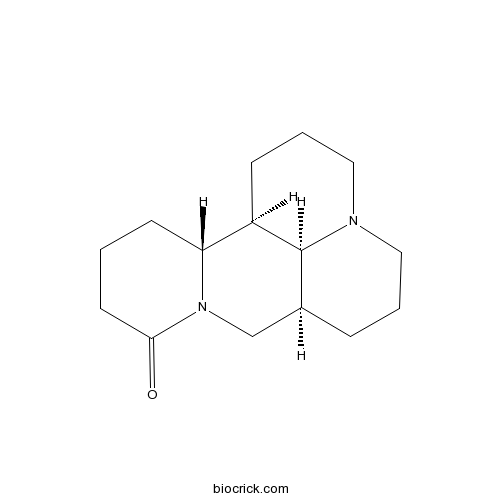
-
BCN5725
Lupeol545-47-1
Instructions

-
BCN4237
Trifolirhizin6807-83-6
Instructions

-
BCN4249
Sophoridine6882-68-4
Instructions

-
BCN2892
(2S)-Isoxanthohumol70872-29-6
Instructions

-
BCN2987
Sophoraflavanone G97938-30-2
Instructions

-
BCN4530
Leachianone A97938-31-3
Instructions

-
BCN2983
Kushenol I99119-69-4
Instructions
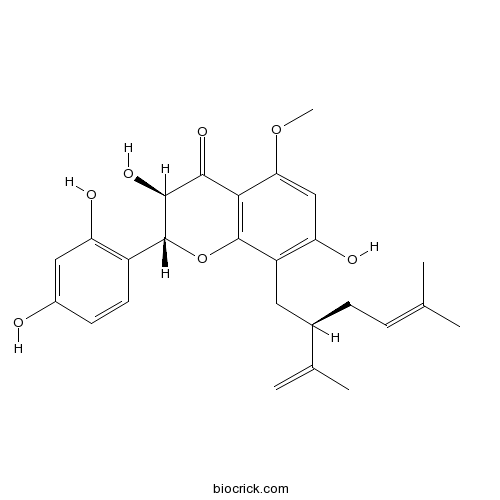
-
BCN2982
Kushenol A99217-63-7
Instructions

Oxymatrine exhibits anti-tumor activity in gastric cancer through inhibition of IL-21R-mediated JAK2/STAT3 pathway.[Pubmed: 30103640]
Oxymatrine (OMT) as a type of alkaloids collected from Sophora flavescens Ait exerts some biological functions including anticancer properties. Here, we investigated the therapeutic effects of OMT in gastric cancer cells (HGC 27 and AGS). As a result, the exposure of gastric cancer (GC) cells to OMT contributed to the suppression of cell proliferation and invasion. Interleukin 21 receptor (IL-21R) was identified to be differentially expressed between OMT treatment group (4 mg/mL) and control group (0 mg/mL), and knockdown of IL-21R repressed cell proliferation and invasion via inactivation of the JAK2/STAT3 pathway. The rescue experiment showed that IL-21R overexpression attenuated the anti-tumor effects of OMT through activation of the JAK2/STAT3 pathway. Moreover, the expression of IL-21R was significantly upregulated in GC samples compared with the adjacent normal tissues and associated with overall survival (OS) and tumor recurrence of GC patients. Taken together, in this study, we evaluated the anti-tumor effects of OMT on GC by investigating proliferation and invasion ability changes, and our findings show that OMT exhibits effects via regulation of JAK/STAT signaling pathway. Through the mechanism study, we may enlighten the potential therapeutic target for treatment of GC.
Direct Activation of the Large-Conductance Calcium-Activated Potassium Channel by Flavonoids Isolated from Sophora flavescens.[Pubmed: 30068881]
None
Symbiotic characteristics of Bradyrhizobium diazoefficiens USDA 110 mutants associated with shrubby sophora (Sophora flavescens) and soybean (Glycine max).[Pubmed: 30031478]
Site-specific insertion plasmid pVO155 was used to knockout the genes involved in the alternation of host range of strain Bradyrhizobium diazoefficiens USDA 110 from its original determinate-nodule-forming host soybean (Glycine max), to promiscuous and indeterminate-nodule-forming shrubby legume sophora (Sophora flavescens). Symbiotic phenotypes of these mutants inoculated to these two legumes, were compared to those infected by wild-type strain USDA 110. Six genes of the total fourteen Tn5 transposon mutated genes were broken using the pVO155 plasmid. Both Tn5 and pVO155-inserted mutants could nodulate S. flavescens with different morphologies of low-efficient indeterminate nodules. One to several rod or irregular bacteroids, containing different contents of poly-β-hydroxybutyrate or polyphosphate were found within the symbiosomes in nodulated cells of S. flavescens infected by the pVO155-inserted mutants. Moreover, none of bacteroids were observed in the pseudonodules of S. flavescens, infected by wild-type strain USDA 110. These mutants had the nodulation ability with soybean but the symbiotic efficiency reduced to diverse extents. These findings enlighten the complicated interactions between rhizobia and legumes, i. e., mutation of genes involved in metabolic pathways, transporters, chemotaxis and mobility could alter the rhizobial entry and development of the bacteroid inside the nodules of a new host legume.
Oxymatrine induces cell cycle arrest and apoptosis and suppresses the invasion of human glioblastoma cells through the EGFR/PI3K/Akt/mTOR signaling pathway and STAT3.[Pubmed: 29989652]
Oxymatrine (OM), a natural quinolizidine alkaloid extracted from the traditional Chinese herb Sophora flavescens, has been revealed to produce antitumor activities in various cancer cell lines, including glioblastoma lines, in vitro. However, the mechanisms by which OM exerts its antitumor effect against glioma are poorly understood. The aim of this study was to investigate the role of OM in the proliferation, apoptosis and invasion of glioma cells and to reveal the underlying mechanisms. The effects of OM on U251MG cells in vitro were determined using a Cell Counting Kit‑8 (CCK‑8) assay, flow cytometric analysis, Annexin V‑FITC/PI staining, DAPI staining, a terminal deoxynucleotidyl transferase‑mediated dUTP nick end‑labeling (TUNEL) assay, a Transwell assay and western blotting. Our data indicated that OM inhibited proliferation, arrested the cell cycle at the G0/G1 phase, decreased the expression levels of G1 cell cycle regulatory proteins (cyclin D1, CDK4 and CDK6), inhibited invasion and induced apoptosis in glioma cells. Additional investigations revealed that the expression levels of p‑STAT3 and key proteins in the EGFR/PI3K/Akt/mTOR signaling pathway, such as p‑EGFR, p‑Akt and p‑mTOR, were markedly decreased after OM treatment, while the total STAT3, EGFR, Akt and mTOR levels were not affected. These findings indicated that the EGFR/PI3K/Akt/mTOR signaling pathway and STAT3 suppression may be a potential mechanism of the OM‑mediated antitumor effect in glioblastoma cells and that EGFR may be a target of OM. Hence, OM may be a promising drug and may offer a novel therapeutic strategy for malignant gliomas in the future.
In vivo and in vitro anti-inflammatory effects of Sophora flavescens residues.[Pubmed: 29913301]
The dried roots of Sophora flavescens Ait. (Leguminosae) is traditionally used as antipyretic medicine to reduce inflammation. It is well known that alkaloids and flavonoids are the main constituents of S. flavescens. However, the clinical researches and applications of S. flavescens is mainly based on its water-extracted alkaloids, its flavonoids may still remain in residues and have been underused. With development and manufacturing of S. flavescens in recent years, more herb residues are being produced. Since they are typically treated as waste and dumped openly in landfill sites, which can cause pollution, there is a great need to explore these wastes as recyclable resources and increase their added value. To date, whether other bioactive components would be found in the residues of S. flavescens is still unknown. If the extraction method of these active ingredients was established, the residues of S. flavescens could be turned from the harm to a benefit and make great sense of the comprehensive utilization of S. flavescens resources. This study aimed to establish an extraction method of the residues of S. flavescens and investigate the anti-inflammatory effect of it both in vivo and in vitro.
Kushenol A and 8-prenylkaempferol, tyrosinase inhibitors, derived from Sophora flavescens.[Pubmed: 29873272]
Tyrosinase is known for an enzyme that plays a key role in producing the initial precursor of melanin biosynthesis. Inhibition of the catalytic reaction of this enzyme led to some advantage such as skin-whitening and anti-insect agents. To find a natural compound with inhibitory activity towards tyrosinase, the five flavonoids of kushenol A (1), 8-prenylkaempferol (2), kushenol C (3), formononetin (4) and 8-prenylnaringenin (5) were isolated by column chromatography from a 95% methanol extract of Sophora flavescens. The ability of these flavonoids to block the conversion of L-tyrosine to L-DOPA by tyrosinase was tested in vitro. Compounds 1 and 2 exhibited potent inhibitory activity, with IC50 values less than 10 µM. Furthermore, enzyme kinetics and molecular docking analysis revealed the formation of a binary encounter complex between compounds 1-4 and the enzyme. Also, all of the isolated compounds (1-5) were confirmed to possess antioxidant activity.
Matrine exerts inhibitory effects in melanoma through the regulation of miR-19b-3p/PTEN.[Pubmed: 29845233]
Matrine, one of the main alkaloid components extracted from the traditional Chinese herb, Sophora flavescens Ait, has various pharmacological effects, and has been reported to exert antitumor activity in melanoma. In the current study, the molecular mechanisms underlying the inhibitory effects of matrine were investigated in melanoma cell line. It was initially confirmed that matrine inhibited proliferation, invasion and induced apoptosis in human A375 and SK-MEL-2 melanoma cell lines in vitro. Subsequently, reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis demonstrated that the expression of microRNA (miR)-19b-3p was significantly increased in melanoma cells and was downregulated by treatment with matrine. Furthermore, downregulated miR-19b-3p exerted effects similar to 500 µg/ml matrine on cell proliferation, invasion and apoptosis. Phosphatase and tensin homolog (PTEN) mRNA was identified as a direct target of miR-19b-3p through bioinformatics analysis and a dual-luciferase reporter assay. Additionally, western blotting and RT-qPCR analysis demonstrated that the expression of PTEN protein and mRNA were increased by the treatment with matrine. Furthermore, silencing of PTEN expression reversed the effects of matrine and miR-19b-3p downregulation in A375 and SK-MEL-2 cells. Taken together, the results indicated that matrine may suppress cell proliferation and invasion and induce cell apoptosis partially via miR-19b-3p targeting of PTEN.
Matrine suppresses KRAS-driven pancreatic cancer growth by inhibiting autophagy-mediated energy metabolism.[Pubmed: 29791786]
Matrine is a natural compound extracted from the herb Sophora flavescens Ait which is widely used in traditional Chinese medicine for treating various diseases. Recently, matrine was reported to have antitumor effects against a variety of cancers without any obvious side effects; however, the molecular mechanisms of its antiproliferative effects on cancer are unclear. Here, we report that matrine inhibits autophagy-mediated energy metabolism, which is necessary for pancreatic cancer growth. We found that matrine significantly reduces pancreatic cancer growth in vitro and in vivo by insufficiently maintaining mitochondrial metabolic function and energy level. We also found that either pyruvate or α-ketoglutarate supplementation markedly rescues pancreatic cancer cell growth following matrine treatment. Inhibition of mitochondrial energy production results from matrine-mediated autophagy inhibition by impairing the function of lysosomal protease. Matrine-mediated autophagy inhibition requires stat3 downregulation. Furthermore, we found that the antitumor effect of matrine on pancreatic cancer growth depends on the mutation of the KRAS oncogene. Together, our data suggest that matrine can suppress the growth of KRAS-mutant pancreatic cancer by inhibiting autophagy-mediated energy metabolism.


