Urena lobata
Urena lobata
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Urena lobata
- Cat.No. Product Name CAS Number COA
-
BCN4889
Tiliroside20316-62-5
Instructions

-
BCN5549
Astragalin480-10-4
Instructions

-
BCN5573
Afzelin482-39-3
Instructions
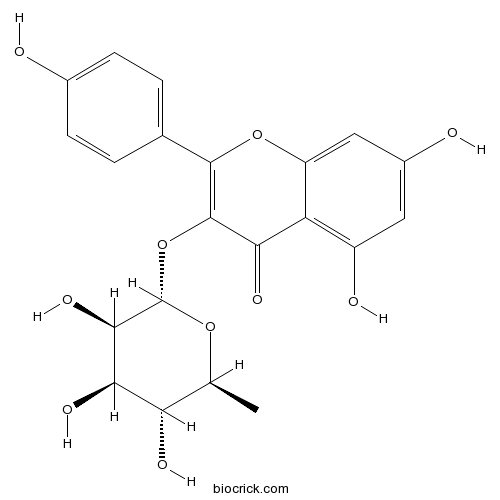
-
BCN5599
Baicalein491-67-8
Instructions
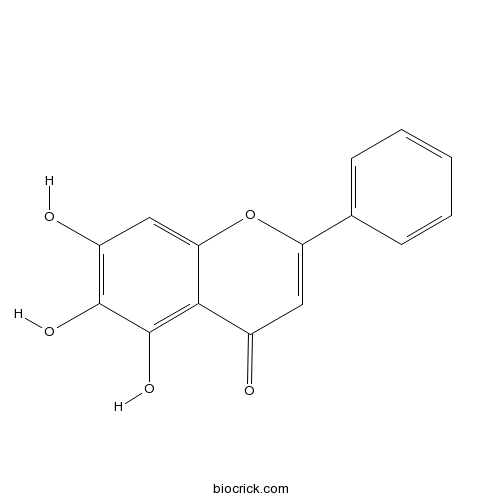
-
BCN5653
Kaempferol520-18-3
Instructions
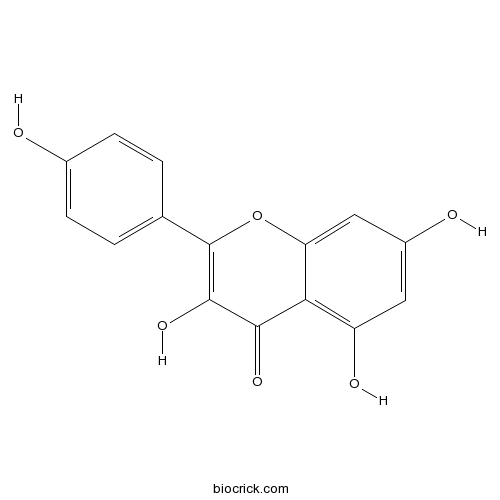
-
BCN5658
Apigenin520-36-5
Instructions

Megastigmane glycosides from Urena lobata.[Pubmed: 29447980]
None
Discovery of a potent anti-yeast triterpenoid saponin, clematoside-S from Urena lobata L.[Pubmed: 25739085]
Urena lobata has been used as a traditional medicinal plant in India and China. In this study, we investigated the antimicrobial activity and isolated the active compound from the leaves of U. lobata. The 80% ethanol extract from U. lobata leaves showed an effective anti-yeast activity against Saccharomyces cerevisiae (S. cerevisiae) strains. Using a combination of chromatographic methods, (-)-trachelogenin (1) and clematoside-S (2) were isolated from this plant for the first time, and their chemical structure was identified by mass spectrometry (MS) and extensive nuclear magnetic resonance (NMR) data analysis. In addition, 1 was found to be inactive against all of the test microorganisms in the antimicrobial assay, whereas 2 exhibits a specific anti-yeast activity against S. cerevisiae strains with diameter of inhibition zones in the range from 11 to 20 mm. Furthermore, the MIC (minimum inhibitory concentration) and MBC (minimum bactericidal concentration) values of 2 against S. cerevisiae strains were detected to be in the ranges of 0.61 to 9.8 μg/mL and 2.42 to 9.8 μg/mL, respectively. This is the first report of 2 with a specific anti-yeast activity. The above result suggests the potential application of U. lobata to be used as a natural anti-yeast agent in food preservation.
Influence of environmental factors on the germination of Urena lobata L. and its response to herbicides.[Pubmed: 24658143]
Urena lobata is becoming a noxious and invasive weed in rangelands, pastures, and undisturbed areas in the Philippines. This study determined the effects of seed scarification, light, salt and water stress, amount of rice residue, and seed burial depth on seed germination and emergence of U. lobata; and evaluated the weed's response to post-emergence herbicides. Germination was stimulated by both mechanical and chemical seed scarifications. The combination of the two scarification methods provided maximum (99%) seed germination. Germination was slightly stimulated when seeds were placed in light (65%) compared with when seeds were kept in the dark (46%). Sodium chloride concentrations ranging from 0 to 200 mM and osmotic potential ranging from 0 to -1.6 MPa affected the germination of U. lobata seeds significantly. The osmotic potential required for 50% inhibition of the maximum germination was -0.1 MPa; however, some seeds germinated at -0.8 MPa, but none germinated at -1.6 MPa. Seedling emergence and biomass increased with increase in rice residue amount up to 4 t ha(-1), but declined beyond this amount. Soil surface placement of weed seeds resulted in the highest seedling emergence (84%), which declined with increase in burial depth. The burial depth required for 50% inhibition of maximum emergence was 2 cm; emergence was greatly reduced (93%) at burial depth of 4 cm or more. Weed seedling biomass also decreased with increase in burial depth. Bispyribac-sodium, a commonly used herbicide in rice, sprayed at the 4-leaf stage of the weed, provided 100% control, which did not differ much with 2,4-D (98%), glyphosate (97%), and thiobencarb + 2,4-D (98%). These herbicides reduced shoot and root biomass by 99-100%.
Preliminary study on antifertility activity of Enicostemma axillare leaves and Urena lobata root used in Indian traditional folk medicine.[Pubmed: 22840449]
To evaluate the possible antifertility activity of Enicostemma axillare (E. axillare) leaves and Urena lobata (U. lobata) root in adult male Wistar albino rats.
Evaluation of Cameroonian plants towards experimental bone regeneration.[Pubmed: 22414477]
Elephantopus mollis, Spilanthes africana, Urena lobata, Momordica multiflora, Asystasia gangetica and Brillantaisia ovariensis are used in Cameroonian traditional medicine for the treatment of bone diseases and fracture repair. The aim of this study was to evaluate the effect of ethanolic extracts of six Cameroonian medicinal plants on bone regeneration following bone and marrow injury.
Three new flavonoid glycosides from Urena lobata.[Pubmed: 21972805]
Three new flavonoid glycosides, kaempferol-3-O-β-D-apiofuranosyl(1 → 2)-β-D-glucopyranosyl-7-O-α-L-rhamnopyranoside (1), kaempferol-4'-O-β-D-apiofuranosyl-3-O-β-D-glucopyranosyl-7-O-α-l-rhamnopyranoside (2), and 5,6,7,4'-tetrahydroxy-flavone-6-O-β-D-arabinopyranosyl-7-O-α-L-rhamnopyranoside (3), were isolated from the aerial parts of Urena lobata L., along with 10 known compounds (4-13). Their structures were determined based on spectroscopic methods including 1D and 2D NMR spectroscopy as well as HR-ESI-MS.
Two new compounds from Urena lobata L.[Pubmed: 21061218]
Two new compounds 1 and 2 have been isolated from the aerial parts of Urena lobata L. The structures of the two new compounds were established as ceplignan-4-O-β-d-glucoside (1) and 2,5-dihydroxy benzoic acid-7-(2,6-dimethyl-6-hydroxy-2,7-octadienoic acid) anhydride-5-O-β-d-apiofuranosyl(1 → 2)-β-d-glucoside (urenoside A) (2), on the basis of chemical and spectral evidence, including 1D and 2D NMR spectroscopic data as well as mass spectrometry (HR-ESI-MS).


