Zea mays
Zea mays
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Zea mays
- Cat.No. Product Name CAS Number COA
-
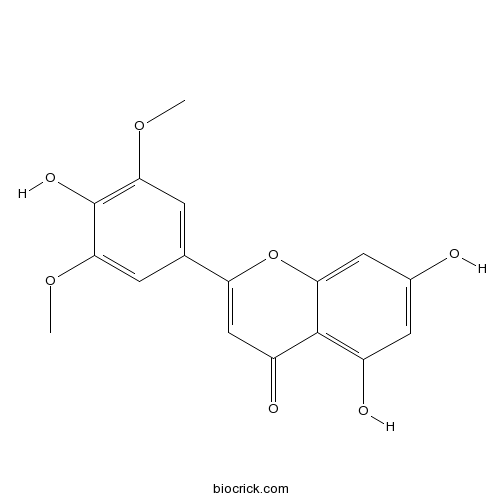
BCN5656
Tricin520-32-1
Instructions
-
BCN1234
Isorhamnetin-3-O-neohespeidoside55033-90-4
Instructions

-
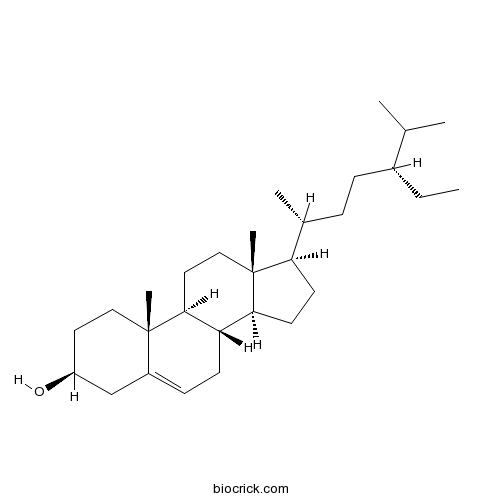
BCN1015
Beta-Sitosterol83-46-5
Instructions
Genetic analysis of seedling root traits reveals the association of root trait with other agronomic traits in maize.[Pubmed: 30111287]
Root systems play important roles in crop growth and stress responses. Although genetic mechanism of root traits in maize (Zea mays L.) has been investigated in different mapping populations, root traits have rarely been utilized in breeding programs. Elucidation of the genetic basis of maize root traits and, more importantly, their connection to other agronomic trait(s), such as grain yield, may facilitate root trait manipulation and maize germplasm improvement. In this study, we analyzed genome-wide genetic loci for maize seedling root traits at three time-points after seed germination to identify chromosomal regions responsible for both seedling root traits and other agronomic traits in a recombinant inbred line (RIL) population (Zong3 × Yu87-1).
In silico characterization and expression profiling of the diacylglycerol acyltransferase gene family (DGAT1, DGAT2, DGAT3 and WS/DGAT) from oil palm, Elaeis guineensis.[Pubmed: 30107884]
The diacylglycerol acyltransferases (DGAT) (diacylglycerol:acyl-CoA acyltransferase, EC 2.3.1.20) are a key group of enzymes that catalyse the final and usually the most important rate-limiting step of triacylglycerol biosynthesis in plants and other organisms. Genes encoding four distinct functional families of DGAT enzymes have been characterised in the genome of the African oil palm, Elaeis guineensis. The contrasting features of the various isoforms within the four families of DGAT genes, namely DGAT1, DGAT2, DGAT3 and WS/DGAT are presented both in the oil palm itself and, for comparative purposes, in 12 other oil crop or model/related plants, namely Arabidopsis thaliana, Brachypodium distachyon, Brassica napus, Elaeis oleifera, Glycine max, Gossypium hirsutum, Helianthus annuus, Musa acuminata, Oryza sativa, Phoenix dactylifera, Sorghum bicolor, and Zea mays. The oil palm genome contains respectively three, two, two and two distinctly expressed functional copies of the DGAT1, DGAT2, DGAT3 and WS/DGAT genes. Phylogenetic analyses of the four DGAT families showed that the E. guineensis genes tend to cluster with sequences from P. dactylifera and M. acuminata rather than with other members of the Commelinid monocots group, such as the Poales which include the major cereal crops such as rice and maize. Comparison of the predicted DGAT protein sequences with other animal and plant DGATs was consistent with the E. guineensis DGAT1 being ER located with its active site facing the lumen while DGAT2, although also ER located, had a predicted cytosol-facing active site. In contrast, DGAT3 and some (but not all) WS/DGAT in E. guineensis are predicted to be soluble, cytosolic enzymes. Evaluation of E. guineensis DGAT gene expression in different tissues and developmental stages suggests that the four DGAT groups have distinctive physiological roles and are particularly prominent in developmental processes relating to reproduction, such as flowering, and in fruit/seed formation especially in the mesocarp and endosperm tissues.
Cooperativity in Plant Plasma Membrane Intrinsic Proteins (PIPs): Mechanism of Increased Water Transport in Maize PIP1 Channels in Hetero-tetramers.[Pubmed: 30104609]
Plant aquaporins (AQPs) play vital roles in several physiological processes. Plasma membrane intrinsic proteins (PIPs) belong to the subfamily of plant AQPs. They are further subdivided into two closely related subgroups PIP1s and PIP2s. While PIP2 members are efficient water channels, PIP1s from some plant species have been shown to be functionally inactive. Aquaporins form tetramers under physiological conditions. PIP2s can enhance the water transport of PIP1s when they form hetero-tetramers. However, the role of monomer-monomer interface and the significance of specific residues in enhancing the water permeation of PIP1s have not been investigated at atomic level. We have performed all-atom molecular dynamics (MD) simulations of homo-tetramers and four different hetero-tetramers containing ZmPIP1;2 and ZmPIP2;5 from Zea mays. ZmPIP1;2 in a tetramer assembly will have two interfaces, one formed by transmembrane segments TM4 and TM5 and the other formed by TM1 and TM2. We have analyzed channel radius profiles, water transport and potential of mean force profiles of ZmPIP1;2 monomers. Results of MD simulations clearly revealed the influence of TM4-TM5 interface in modulating the water transport of ZmPIP1;2. MD simulations indicate the importance of I93 residue from the TM2 segment of ZmPIP2;5 for the increased water transport in ZmPIP1;2.
Heterosis-related genes under different planting densities in maize (Zea mays L.).[Pubmed: 30085089]
Heterosis and increasing planting density have contributed to improving maize grain yield (GY) for several decades. As planting densities increase, the GY per plot also increases whereas the contribution of heterosis to GY decreases. There are trade-offs between heterosis and planting density, and the transcriptional characterization of heterosis may explain the mechanism involved. In this study, 48 transcriptome libraries were sequenced from four inbred Chinese maize lines and their F1 hybrids. They were planted at densities of 45,000 plants/ha and 67,500 plants/ha. Maternal-effect differentially expressed genes (DEGs) played important roles in processes related to photosynthesis and carbohydrate biosynthesis and metabolism. Paternal-effect DEGs participated in abiotic/biotic stress response and plant hormone production under high planting density. Weighted gene co-expression network analysis revealed that high planting-density induced heterosis-related genes regulating abiotic/biotic stress response, plant hormone biosynthesis, and ubiquitin-mediated proteolysis but repressed other genes regulating energy formation. Under high planting density, maternal genes were mainly enriched in the photosynthesis reaction center, while paternal genes were mostly concentrated in the peripheral antenna system. Four important genes were identified in maize heterosis and high planting density, all with functions in photosynthesis, starch biosynthesis, auxin metabolism, gene silencing, and RNA interference.


