TricinCAS# 520-32-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 520-32-1 | SDF | Download SDF |
| PubChem ID | 5281702 | Appearance | Light yellow powder |
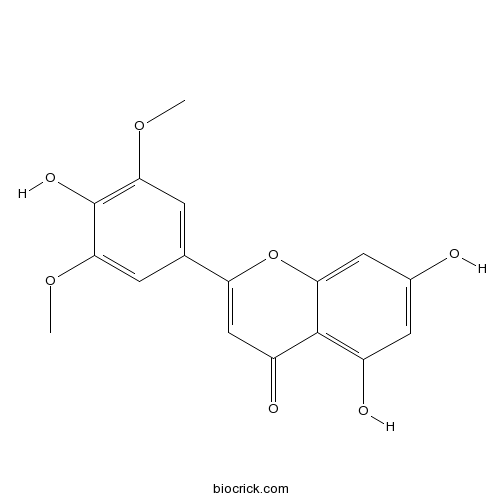
| Formula | C17H14O7 | M.Wt | 330.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | 4',5,7-Trihydroxy 3',5'-dimethoxyflavone | ||
| Solubility | DMSO : 125 mg/mL (378.46 mM; Need ultrasonic) | ||
| Chemical Name | 5,7-dihydroxy-2-(4-hydroxy-3,5-dimethoxyphenyl)chromen-4-one | ||
| SMILES | COC1=CC(=CC(=C1O)OC)C2=CC(=O)C3=C(C=C(C=C3O2)O)O | ||
| Standard InChIKey | HRGUSFBJBOKSML-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H14O7/c1-22-14-3-8(4-15(23-2)17(14)21)12-7-11(20)16-10(19)5-9(18)6-13(16)24-12/h3-7,18-19,21H,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Tricin is evaluated as a type of tyrosinase inhibitor. 2. Tricin derivatives conveys allergy and inflammation treatment ability to Z. latifolia. 3. Tricin exerts anti-inflammatory effect via a mechanism involving the TLR4/NF-κB/STAT signaling cascade. 4. Tricin may be beneficial in HSC-targeting therapeutic or chemopreventive applications for hepatic fibrosis. 5. Tricin is a novel compound with potential anti-HCMV activity and that CXCL11 is one of the chemokines involved in HCMV replication. |
| Targets | TLR | p38MAPK | JNK | NF-kB | Phospholipase (e.g. PLA) | Tyrosinase | COX | JAK | STAT | Antifection |

Tricin Dilution Calculator

Tricin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0276 mL | 15.1378 mL | 30.2755 mL | 60.551 mL | 75.6888 mL |
| 5 mM | 0.6055 mL | 3.0276 mL | 6.0551 mL | 12.1102 mL | 15.1378 mL |
| 10 mM | 0.3028 mL | 1.5138 mL | 3.0276 mL | 6.0551 mL | 7.5689 mL |
| 50 mM | 0.0606 mL | 0.3028 mL | 0.6055 mL | 1.211 mL | 1.5138 mL |
| 100 mM | 0.0303 mL | 0.1514 mL | 0.3028 mL | 0.6055 mL | 0.7569 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Tectochrysin
Catalog No.:BCN5655
CAS No.:520-28-5
- Diosimin
Catalog No.:BCN4993
CAS No.:520-27-4
- Hesperidin
Catalog No.:BCN5654
CAS No.:520-26-3
- Kaempferol
Catalog No.:BCN5653
CAS No.:520-18-3
- Pectolinarigenin
Catalog No.:BCN5813
CAS No.:520-12-7
- 6-Methoxyluteolin
Catalog No.:BCN3613
CAS No.:520-11-6
- H-Cys-OH
Catalog No.:BCC2902
CAS No.:52-90-4
- Haloperidol
Catalog No.:BCC4909
CAS No.:52-86-8
- Lynestrenol
Catalog No.:BCC9014
CAS No.:52-76-6
- H-D-Pen-OH
Catalog No.:BCC3307
CAS No.:52-67-5
- Morphine hydrochloride
Catalog No.:BCC6368
CAS No.:52-26-6
- Thio-TEPA
Catalog No.:BCC5354
CAS No.:52-24-4
- Hesperetin
Catalog No.:BCN5657
CAS No.:520-33-2
- Diosmetin
Catalog No.:BCN2356
CAS No.:520-34-3
- Apigenin
Catalog No.:BCN5658
CAS No.:520-36-5
- Asebogenin
Catalog No.:BCN7232
CAS No.:520-42-3
- Psilocin
Catalog No.:BCC6168
CAS No.:520-53-6
- Spectabiline
Catalog No.:BCN2098
CAS No.:520-55-8
- Spartioidine
Catalog No.:BCN2134
CAS No.:520-59-2
- Rosmarinine
Catalog No.:BCN2124
CAS No.:520-65-0
- Echimidine
Catalog No.:BCN1967
CAS No.:520-68-3
- Medroxyprogesterone
Catalog No.:BCC5231
CAS No.:520-85-4
- Isoschaftoside
Catalog No.:BCN3011
CAS No.:52012-29-0
- NCS-382
Catalog No.:BCC6794
CAS No.:520505-01-5
Mechanism of anti-inflammatory effect of tricin, a flavonoid isolated from Njavara rice bran in LPS induced hPBMCs and carrageenan induced rats.[Pubmed:25839778]
Mol Immunol. 2015 Aug;66(2):229-39.
Njavara is an indigenous medicinal rice variety traditionally used in Ayurvedic system of medicine practiced in Kerala, India. Tricin is a bioflavonoid present in significantly higher levels in rice bran of Njavara. Present study attempted to identify the molecular target of Tricin in TLR mediated signaling pathways by using lipopolysaccharide (LPS) induced human peripheral blood mononuclear cells (hPBMCs) and carrageenan induced paw edema in rats as experimental models. Tricin acted upstream in the activation of inflammation cascade by interfering with TLR4 activation, preferably by blocking the LPS induced activation of TLR4, MYD88 and TRIF proteins in hPBMCs. Subsequently, Tricin significantly blocked the activation of downstream kinases like p38MAPK, JNK1/2 and IRF3. Thus the inhibitory effect of Tricin on NF-kappaB and IRF3 together confirms the specific inhibition of both MYD88 dependent and TRIF dependent pathways. Tricin treatment also inhibited the pro-inflammatory effect of LPS by blocking the TLR4 signaling mediated activation of cytosolic phospholipase A2 (cPLA2), which is confirmed by specific inhibition of COX-2. Results demonstrated that in addition to NF-kappaB, Tricin can prevent the activation of STAT proteins by significantly inhibiting the activation of both STAT1 and STAT3 via the down regulation of upstream phosphorylating enzymes like JAK1 and JAK2. The protective anti-inflammatory effect of Tricin was also confirmed by in vivo experiments. Thus, this study provides strong evidence that Tricin exerts its anti-inflammatory effect via a mechanism involving the TLR4/NF-kappaB/STAT signaling cascade.
Molecular inhibitory mechanism of tricin on tyrosinase.[Pubmed:23434549]
Spectrochim Acta A Mol Biomol Spectrosc. 2013 Apr 15;107:235-40.
Tricin was evaluated as a type of tyrosinase inhibitor with good efficacy compared to arbutin. Tricin functioned as a non-competitive inhibitor of tyrosinase, with an equilibrium constant of 2.30 mmol/L. The molecular mechanisms underlying the inhibition of tyrosinase by Tricin were investigated by means of circular dichroism spectra, fluorescence quenching and molecular docking. These assays demonstrated that the interactions between Tricin and tyrosinase did not change the secondary structure. The interaction of Tricin with residues in the hydrophobic pocket of tyrosinase was revealed by fluorescence quenching; the complex was stabilized by hydrophobic associations and hydrogen bonding (with residues Asn80 and Arg267). Docking results implied that the possible inhibitory mechanisms may be attributed to the stereospecific blockade effects of Tricin on substrates or products and flexible conformation alterations in the tyrosinase active center caused by weak interactions between tyrosinase and Tricin. The application of this type of flavonoid as a tyrosinase inhibitor will lead to significant advances in the field of depigmentation.
Tricin derivatives as anti-inflammatory and anti-allergic constituents from the aerial part of Zizania latifolia.[Pubmed:25559019]
Biosci Biotechnol Biochem. 2015;79(5):700-6.
Methanol extract of Zizania latifolia was partitioned with EtOAc, n-BuOH, and H2O. From the EtOAc layers, a new flavonolignan along with a known flavone and three known flavonolignans, Tricin (1), salcolin A (2), salcolin B (3), and salcolin C (4), were isolated through repeated silica gel and ODS column chromatography. The chemical structure of the new flavonolignan was determined to be Tricin-4'-O-[erythro-beta-guaiacyl-(7''-O-methyl)-glyceryl] ether and was named salcolin D (5) based on physicochemical and spectroscopic data, including FT-NMR and ESI-MS. All compounds were isolated for the first time from this plant. Compounds 2-5, Tricin derivatives, all exhibited higher anti-inflammatory and anti-allergy activities than Tricin. In particular, salcolin D (5) was shown to have the strongest inhibitory activity against LPS-induced NO production in RAW 264.7 cells as well as beta-hexosaminidase release in IgE-sensitized RBL-2H3 cells. These results suggest that the presence of Tricin derivatives conveys allergy and inflammation treatment ability to Z. latifolia.
Anti-cytomegalovirus effects of tricin are dependent on CXCL11.[Pubmed:22683667]
Microbes Infect. 2012 Oct;14(12):1086-92.
It has been reported that treatment with Tricin (4',5,7-trihydroxy-3',5'-dimethoxyflavone), a derivative of Sasa albo-marginata, after human cytomegalovirus (HCMV) infection significantly suppressed both infectious virus production and HCMV replication in the human embryonic fibroblast cell line MRC-5. In this paper, we examined the mechanisms for the anti-HCMV effects of Tricin in MRC-5 cells. Exposure of fibroblasts to Tricin inhibited infectious HCMV production, with concomitant decreases in levels of transcripts of the CXC chemokine IFN-inducible T cell alpha chemoattractant (I-TAC or CXCL11) gene. We also found that the transcripts of the HCMV immediate early (IE) gene and replication of HCMV were lower in CXCL11 gene-knockdown cells. These results suggest that Tricin is a novel compound with potential anti-HCMV activity and that CXCL11 is one of the chemokines involved in HCMV replication. In addition, it is possible that CXCL11 is the one of the targets of Tricin.


