TectochrysinCAS# 520-28-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 520-28-5 | SDF | Download SDF |
| PubChem ID | 5281954 | Appearance | White powder |
| Formula | C16H12O4 | M.Wt | 268.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | 5-Hydroxy 7-methoxyflavone; 7-Methylchrysin | ||
| Solubility | DMSO : 16.67 mg/mL (62.14 mM; Need ultrasonic) | ||
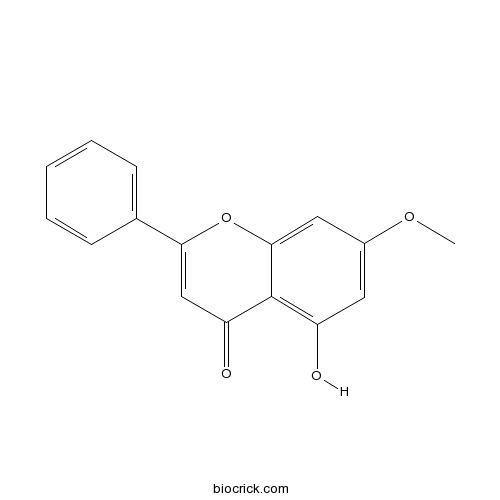
| Chemical Name | 5-hydroxy-7-methoxy-2-phenylchromen-4-one | ||
| SMILES | COC1=CC(=C2C(=C1)OC(=CC2=O)C3=CC=CC=C3)O | ||
| Standard InChIKey | IRZVHDLBAYNPCT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H12O4/c1-19-11-7-12(17)16-13(18)9-14(20-15(16)8-11)10-5-3-2-4-6-10/h2-9,17H,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tectochrysin is a promising inhibitor for the reversal of ABCG2-mediated drug transport, it leads to apoptotic cell death in NSCLC cells through activation of DR3 and Fas expression via inhibition of STAT3 phosphorylation.Tectochrysin has antioxidant effect, it also exhibits a significant hepatoprotective activity in hepatic damage induced by CCl4-intoxication in rats. |
| Targets | STAT | Caspase | Bcl-2/Bax | ATPase |
| In vivo | In vivo anti-oxidant activities of tectochrysin.[Pubmed: 12568357]Arch Pharm Res. 2003 Jan;26(1):43-6.The anti-oxidant activities of Tectochrysin, a major compound of propolis, were investigated.
|
| Kinase Assay | Anti-cancer effect of tectochrysin in NSCLC cells through overexpression of death receptor and inactivation of STAT3.[Pubmed: 25083589]Cancer Lett. 2014 Oct 10;353(1):95-103.Phenolic compounds (flavonoids and phenolic acid derivatives) are the most important pharmacologically active ingredients, and these compounds could inhibit proliferation of human cancer cells by inducing of apoptotic cell death.
|
| Structure Identification | Cancer Res. 2005 Jun 1;65(11):4852-60.Flavonoid structure-activity studies identify 6-prenylchrysin and tectochrysin as potent and specific inhibitors of breast cancer resistance protein ABCG2.[Pubmed: 15930306]Overexpression of breast cancer resistance protein ABCG2 confers multidrug resistance in cancer cells. The GF120918-sensitive drug efflux activity of human wild-type (R482) ABCG2-transfected cells was used for rational screening of inhibitory flavonoids and establishment of structure-activity relationships.
|

Tectochrysin Dilution Calculator

Tectochrysin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7272 mL | 18.6359 mL | 37.2717 mL | 74.5434 mL | 93.1793 mL |
| 5 mM | 0.7454 mL | 3.7272 mL | 7.4543 mL | 14.9087 mL | 18.6359 mL |
| 10 mM | 0.3727 mL | 1.8636 mL | 3.7272 mL | 7.4543 mL | 9.3179 mL |
| 50 mM | 0.0745 mL | 0.3727 mL | 0.7454 mL | 1.4909 mL | 1.8636 mL |
| 100 mM | 0.0373 mL | 0.1864 mL | 0.3727 mL | 0.7454 mL | 0.9318 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Diosimin
Catalog No.:BCN4993
CAS No.:520-27-4
- Hesperidin
Catalog No.:BCN5654
CAS No.:520-26-3
- Kaempferol
Catalog No.:BCN5653
CAS No.:520-18-3
- Pectolinarigenin
Catalog No.:BCN5813
CAS No.:520-12-7
- 6-Methoxyluteolin
Catalog No.:BCN3613
CAS No.:520-11-6
- H-Cys-OH
Catalog No.:BCC2902
CAS No.:52-90-4
- Haloperidol
Catalog No.:BCC4909
CAS No.:52-86-8
- Lynestrenol
Catalog No.:BCC9014
CAS No.:52-76-6
- H-D-Pen-OH
Catalog No.:BCC3307
CAS No.:52-67-5
- Morphine hydrochloride
Catalog No.:BCC6368
CAS No.:52-26-6
- Thio-TEPA
Catalog No.:BCC5354
CAS No.:52-24-4
- Prednisolone Acetate
Catalog No.:BCC4831
CAS No.:52-21-1
- Tricin
Catalog No.:BCN5656
CAS No.:520-32-1
- Hesperetin
Catalog No.:BCN5657
CAS No.:520-33-2
- Diosmetin
Catalog No.:BCN2356
CAS No.:520-34-3
- Apigenin
Catalog No.:BCN5658
CAS No.:520-36-5
- Asebogenin
Catalog No.:BCN7232
CAS No.:520-42-3
- Psilocin
Catalog No.:BCC6168
CAS No.:520-53-6
- Spectabiline
Catalog No.:BCN2098
CAS No.:520-55-8
- Spartioidine
Catalog No.:BCN2134
CAS No.:520-59-2
- Rosmarinine
Catalog No.:BCN2124
CAS No.:520-65-0
- Echimidine
Catalog No.:BCN1967
CAS No.:520-68-3
- Medroxyprogesterone
Catalog No.:BCC5231
CAS No.:520-85-4
- Isoschaftoside
Catalog No.:BCN3011
CAS No.:52012-29-0
In vivo anti-oxidant activities of tectochrysin.[Pubmed:12568357]
Arch Pharm Res. 2003 Jan;26(1):43-6.
The anti-oxidant activities of Tectochrysin, a major compound of propolis, were investigated. Tectochrysin exhibited a significant decrease in serum transaminase activities elevated by hepatic damage induced by CCl4-intoxication in rats. Tectochrysin tested exhibited a lipid peroxidation causing a significant decrease in MDA production in TBA-reactant assay. Tectochrysin was strong in the increase in the anti-oxidant enzymes such as hepatic cytosolic superoxide dismutase, catalase and glutathione peroxidase activities in CCl4-intoxicated rats. These results suggest that Tectochrysin possess not only the anti-oxidant, but also the activities in CCl4-intoxicated rats. Especially, Tectochrysin was found to cause significant increases in the rat liver cytosolic SOD, catalase, GSH-px activities as well as a significant decrease in the MDA production.
Flavonoid structure-activity studies identify 6-prenylchrysin and tectochrysin as potent and specific inhibitors of breast cancer resistance protein ABCG2.[Pubmed:15930306]
Cancer Res. 2005 Jun 1;65(11):4852-60.
Overexpression of breast cancer resistance protein ABCG2 confers multidrug resistance in cancer cells. The GF120918-sensitive drug efflux activity of human wild-type (R482) ABCG2-transfected cells was used for rational screening of inhibitory flavonoids and establishment of structure-activity relationships. Flavones were found more efficient than flavonols, isoflavones, and flavanones. Differentially substituted flavone derivatives indicated positive OH effects at position 5, in contrast to positions 3 and 7. A methoxy at position 7 was slightly positive in Tectochrysin, whereas a strong positive effect was produced by prenylation at position 6. The potency of 6-prenylchrysin was comparable with that of GF120918 (IC50 = 0.3 micromol/L). Both 6-prenylchrysin and Tectochrysin seemed specific for ABCG2 because no interaction was detected with either P-glycoprotein or MRP1. The ABCG2 resistance profile in vitro is altered by mutation at amino acid 482. The R482T mutation limited the effect of prenylation on ABCG2 inhibition. Whereas GF120918 strongly inhibited the ATPase activity of wild-type ABCG2, neither 6-prenylchrysin nor Tectochrysin altered the activity. In contrast, all three inhibitors stimulated the ATPase activity of mutant ABCG2. 6-Prenylchrysin at 0.5 micromol/L efficiently sensitized the growth of wild-type ABCG2-transfected cells to mitoxantrone, whereas higher concentrations were required for the mutant ones. In contrast, 1 micromol/L Tectochrysin was sufficient to fully sensitize mutant ABCG2-transfected cells, whereas higher concentrations were required for the wild-type ones. Both flavones exhibited a lower intrinsic cytotoxicity than GF120918 and were apparently not transported by ABCG2. 6-Prenylchrysin and Tectochrysin therefore constitute new and promising inhibitors for the reversal of ABCG2-mediated drug transport.
Anti-cancer effect of tectochrysin in NSCLC cells through overexpression of death receptor and inactivation of STAT3.[Pubmed:25083589]
Cancer Lett. 2014 Oct 10;353(1):95-103.
Phenolic compounds (flavonoids and phenolic acid derivatives) are the most important pharmacologically active ingredients, and these compounds could inhibit proliferation of human cancer cells by inducing of apoptotic cell death. Here we focused on the anticancer effects of Tectochrysin on human non-small-cell lung cancer (NSCLC) cells and its mechanism of action. We analysed the activity of Tectochrysin on NSCLC cells (A549 and NCI-H460) by use of Western blot analysis for major apoptotic proteins and death receptor expression. We also used EMSA for effects on STAT3 DNA binding activity. Tectochrysin (0-80 muM) suppressed the growth of A549 and NCI-H460 lung cancer cells by inducing of apoptotic cell death in a concentration dependent manner. Expression of DR3 and Fas as well as DR downstream pro-apoptotic proteins including cleaved caspase-3, cleaved caspase-8, cleaved caspase-9 and Bax were concomitantly increased, but the expression of anti-apoptotic proteins; Bcl-2 was decreased in both cancer cells. In addition, Tectochrysin treatment also inhibited phosphorylation of STAT3 in A549 and NCI-H460 cells. However, deletion of DR3 and Fas by small interfering RNA significantly reversed Tectochrysin-induced cell growth inhibitory effect as well as down regulation of STAT3 in A549 and NCI-H460 lung cancer cells. Pull down assay and docking model showed interaction of Tectochrysin with STAT3. We propose that Tectochrysin leads to apoptotic cell death in NSCLC cells through activation of DR3 and Fas expression via inhibition of STAT3 phosphorylation.


