AsebogeninCAS# 520-42-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 520-42-3 | SDF | Download SDF |
| PubChem ID | 442255 | Appearance | Powder |
| Formula | C16H16O5 | M.Wt | 288.29 |
| Type of Compound | Chalcones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
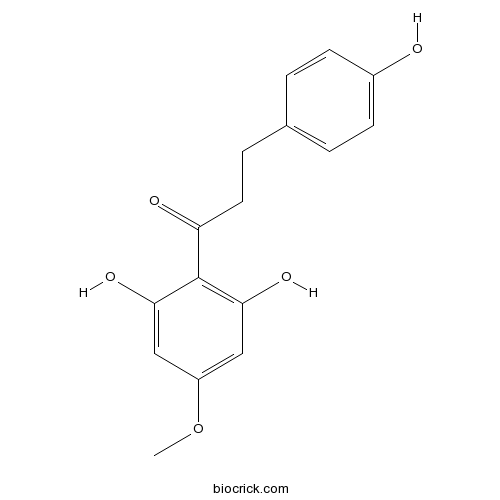
| Chemical Name | 1-(2,6-dihydroxy-4-methoxyphenyl)-3-(4-hydroxyphenyl)propan-1-one | ||
| SMILES | COC1=CC(=C(C(=C1)O)C(=O)CCC2=CC=C(C=C2)O)O | ||
| Standard InChIKey | UPXIBKPHJYQSGP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H16O5/c1-21-12-8-14(19)16(15(20)9-12)13(18)7-4-10-2-5-11(17)6-3-10/h2-3,5-6,8-9,17,19-20H,4,7H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Asebogenin shows antiplasmodial activities against both a chloroquine-sensitive and a resistant strain of Plasmodium falciparum. 2. Asebogenin can inhibit the proliferation of murine B cells. 3. Asebogenin has anti-bacterial activity, it shows inhibitory activity against S. aureus and methicillin-resistant S. aureus (IC50 of 10 and 4.5 micrograms/ml, respectively) . |
| Targets | ATPase | Potassium Channel | Antifection |

Asebogenin Dilution Calculator

Asebogenin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4687 mL | 17.3436 mL | 34.6873 mL | 69.3746 mL | 86.7182 mL |
| 5 mM | 0.6937 mL | 3.4687 mL | 6.9375 mL | 13.8749 mL | 17.3436 mL |
| 10 mM | 0.3469 mL | 1.7344 mL | 3.4687 mL | 6.9375 mL | 8.6718 mL |
| 50 mM | 0.0694 mL | 0.3469 mL | 0.6937 mL | 1.3875 mL | 1.7344 mL |
| 100 mM | 0.0347 mL | 0.1734 mL | 0.3469 mL | 0.6937 mL | 0.8672 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Apigenin
Catalog No.:BCN5658
CAS No.:520-36-5
- Diosmetin
Catalog No.:BCN2356
CAS No.:520-34-3
- Hesperetin
Catalog No.:BCN5657
CAS No.:520-33-2
- Tricin
Catalog No.:BCN5656
CAS No.:520-32-1
- Tectochrysin
Catalog No.:BCN5655
CAS No.:520-28-5
- Diosimin
Catalog No.:BCN4993
CAS No.:520-27-4
- Hesperidin
Catalog No.:BCN5654
CAS No.:520-26-3
- Kaempferol
Catalog No.:BCN5653
CAS No.:520-18-3
- Pectolinarigenin
Catalog No.:BCN5813
CAS No.:520-12-7
- 6-Methoxyluteolin
Catalog No.:BCN3613
CAS No.:520-11-6
- H-Cys-OH
Catalog No.:BCC2902
CAS No.:52-90-4
- Haloperidol
Catalog No.:BCC4909
CAS No.:52-86-8
- Psilocin
Catalog No.:BCC6168
CAS No.:520-53-6
- Spectabiline
Catalog No.:BCN2098
CAS No.:520-55-8
- Spartioidine
Catalog No.:BCN2134
CAS No.:520-59-2
- Rosmarinine
Catalog No.:BCN2124
CAS No.:520-65-0
- Echimidine
Catalog No.:BCN1967
CAS No.:520-68-3
- Medroxyprogesterone
Catalog No.:BCC5231
CAS No.:520-85-4
- Isoschaftoside
Catalog No.:BCN3011
CAS No.:52012-29-0
- NCS-382
Catalog No.:BCC6794
CAS No.:520505-01-5
- H-Val-pNA
Catalog No.:BCC3139
CAS No.:52084-13-6
- 2-Hydroxy-7-O-methylscillascillin
Catalog No.:BCN5659
CAS No.:52096-50-1
- Mestanolone
Catalog No.:BCC9022
CAS No.:521-11-9
- Dromostanolone propionate
Catalog No.:BCC8954
CAS No.:521-12-0
Dihydrochalcones from the leaves of Pieris japonica.[Pubmed:15787442]
J Nat Prod. 2005 Mar;68(3):392-6.
Six new dihydrochalcones, 3-hydroxyasebotin (5), Asebogenin 2'-O-beta-D-ribohexo-3-ulopyranoside (6), 2' '-acetylasebotin (7), 3',4,5'-trihydroxy-4'-methoxydihydrochalcone 3',5'-di-O-beta-D-glucopyranoside (8), and pierotins A (9) and B (10), along with four known dihydrochalcones, phloretin (1), phlorizin (2), Asebogenin (3), and asebotin (4), were isolated from the leaves of Pieris japonica. Their structures were elucidated on the basis of spectroscopic analysis including HMQC, HMBC, NOESY, and X-ray crystal diffraction. Compounds 1, 3-5, and 7-10 inhibited the proliferation of murine B cells and compounds 5 and 10 inhibited the proliferation of murine T cells in vitro significantly.
Dihydrochalcones from Piper longicaudatum.[Pubmed:11301876]
Planta Med. 2001 Mar;67(2):186-8.
Bioactivity-guided fractionation of an ethanolic extract of the leaves and twigs of Piper longicaudatum Trelease & Yunker (Piperaceae) resulted in the isolation of one new (1) and three known (2-4) dihydrochalcones. The known compounds are: 2',6'-dihydroxy-4'-methoxydihydrochalcone (2), 2',6',4-trihydroxy-4'-methoxydihydrochalcone (Asebogenin) (3), and 2'-hydroxy-4'-methoxy-2'-[1-hydroxy-1-methylethyl]-2",3"-dihy- drofurano[4",5":5',6"]-3"-[2-hydroxy-5-methoxycarbonylphe- nyl]dihydrochalcone (piperaduncin B) (4). The new compound is 2'-hydroxy-4'-methoxy-2"-[2-hydroxy-5-methoxycarbonyl- phenyl]-furano[4",5":5',6']-dihydrochalcone (longicaudatin) (1). Compounds 1-4 were tested for antibacterial activity against S. aureus and methicillin-resistant S. aureus (MRSA); only compound 3 showed inhibitory activity (IC50 of 10 and 4.5 micrograms/ml, respectively).
Anti-secretory, anti-inflammatory and anti-Helicobacter pylori activities of several fractions isolated from Piper carpunya Ruiz & Pav.[Pubmed:20152892]
J Ethnopharmacol. 2010 Apr 21;128(3):583-9.
ETHNOPHARMACOLOGICAL RELEVANCE: The leaves of Piper carpunya Ruiz & Pav. (syn Piper lenticellosum C.D.C.) (Piperaceae), are widely used in folk medicine in tropical and subtropical countries of South America as an anti-inflammatory, anti-ulcer, anti-diarrheal and anti-parasitical remedy as well as an ailment for skin irritations. AIMS OF THE STUDY: To study the anti-inflammatory, anti-secretory and anti-Helicobacter pylori activities of different fractions isolated from an ethanolic extract of the leaves of Piper carpunya, in order to provide evidence for the use of this plant as an anti-ulcer remedy. Moreover, to isolate the main compounds of the extract and relate their biological activity to the experimental results obtained with the fractions. MATERIALS AND METHODS: Sixteen fractions were obtained from the ethanolic extract (F I-XVI) and 16 pure compounds were isolated and identified from these fractions. We studied the effects of the fractions (0.1-400microg/mL) on the release of myeloperoxidase (MPO) enzyme from rat peritoneal leukocytes, on rabbit gastric microsomal H(+), K(+)-ATPase activity and anti-Helicobacter pylori anti-microbial activity using the microdilution method (MM). The main compounds contained in the fractions were isolated and identified by (1)H- and (13)C NMR spectra analysis and comparison with the literature data. RESULTS: Eight fractions showed inhibition of MPO enzyme (F I-IV, X, XII, XIV and XV). The highest inhibition was observed with F XIV (50microg/mL, 60.9%, p<0.001). F X and XII were the most active ones, inhibiting the gastric H(+), K(+)-ATPase activity with IC(50) values equal to 22.3microg/mL and 28.1microg/mL, respectively. All fractions, except F XV, presented detectable anti-Helicobacter pylori activity, with a diameter of inhibition zones ranging from 11mm up to 50mm. The best anti-Helicobacter pylori activity was obtained with F III and V. Both fractions killed Helicobacter pylori with lowest concentration values, about 6.25mug/mL. Sixteen pure compounds were isolated, five of them are flavonoids that possess strong anti-oxidant and free radical scavenging activity, e.g. vitexin, isovitexin, and rhamnopyranosylvitexin. Terpenoids like sitosterol, stigmasterol and phytol, which have shown gastroprotective activity, and dihydrochalcones, like Asebogenin, with anti-bacterial activity, were also isolated. Furthermore, the rare neolignan 1, that is a DNA polymerase beta lyase inhibitor, and (6S, 9S)-roseoside, that shows strong anti-bacterial activity, were isolated, for the first time, from the genus Piper. CONCLUSIONS: We suggest that the flavonoids isolated from F I and II (vitexin, isovitexin, rhamnopyranosylvitexin and isoembigenin) contribute to the anti-MPO activity, as well as to their anti-Helicobacter pylori activity. These flavonoids may also be responsible for the important inhibition of H(+), K(+)-ATPase activity. Also the phytosterols and phytol obtained from F XIV and XV could be involved in these gastroprotective activities. These results encourage us to continue phytochemical studies on these fractions in order to obtain full scientific validation for this species.
In vitro antiplasmodial activity of Central American medicinal plants.[Pubmed:10540301]
Trop Med Int Health. 1999 Sep;4(9):611-5.
The in vitro antiplasmodial activities of 14 plant species traditionally used in Central America for the treatment of malaria or fever were evaluated. Lipophilic extracts of Piper hispidum, Siparuna andina, S. pauciflora, S. tonduziana, and Xylopia cf. frutescens, proved to be active against both a chloroquine-sensitive and a resistant strain of Plasmodium falciparum. IC50 values ranged between 3.0 microg/ml and 21.9 microg/ml; however, moderate cytotoxicity of active extracts was observed. Bioactivity-guided fractionation of Piper hispidum yielded 2',4, 6'-trihydroxy-4'-methoxydihydrochalcone (Asebogenin) as an active compound.


