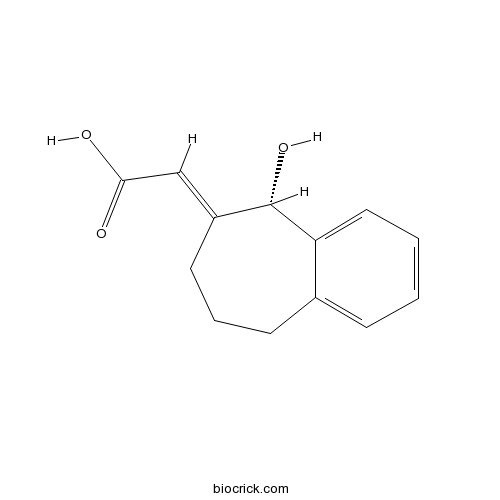
NCS-382CAS# 520505-01-5 |
- Limonin
Catalog No.:BCN6057
CAS No.:1180-71-8
- Fosamprenavir Calcium Salt
Catalog No.:BCC1581
CAS No.:226700-81-8
- HIV-1 integrase inhibitor
Catalog No.:BCC1618
CAS No.:544467-07-4
- BMS-626529
Catalog No.:BCC1427
CAS No.:701213-36-7
- HIV-1 integrase inhibitor 2
Catalog No.:BCC1619
CAS No.:957890-42-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 520505-01-5 | SDF | Download SDF |
| PubChem ID | 6604757 | Appearance | Powder |
| Formula | C13H14O3 | M.Wt | 218.25 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
| Chemical Name | (2E)-2-[(5S)-5-hydroxy-5,7,8,9-tetrahydrobenzo[7]annulen-6-ylidene]acetic acid | ||
| SMILES | C1CC2=CC=CC=C2C(C(=CC(=O)O)C1)O | ||
| Standard InChIKey | UADPGHINQMWEAG-FROQITRMSA-N | ||
| Standard InChI | InChI=1S/C13H14O3/c14-12(15)8-10-6-3-5-9-4-1-2-7-11(9)13(10)16/h1-2,4,7-8,13,16H,3,5-6H2,(H,14,15)/b10-8+/t13-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | γ-Hydroxybutyric acid antagonist, anticonvulsant. |

NCS-382 Dilution Calculator

NCS-382 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.5819 mL | 22.9095 mL | 45.819 mL | 91.638 mL | 114.5475 mL |
| 5 mM | 0.9164 mL | 4.5819 mL | 9.1638 mL | 18.3276 mL | 22.9095 mL |
| 10 mM | 0.4582 mL | 2.291 mL | 4.5819 mL | 9.1638 mL | 11.4548 mL |
| 50 mM | 0.0916 mL | 0.4582 mL | 0.9164 mL | 1.8328 mL | 2.291 mL |
| 100 mM | 0.0458 mL | 0.2291 mL | 0.4582 mL | 0.9164 mL | 1.1455 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isoschaftoside
Catalog No.:BCN3011
CAS No.:52012-29-0
- Medroxyprogesterone
Catalog No.:BCC5231
CAS No.:520-85-4
- Echimidine
Catalog No.:BCN1967
CAS No.:520-68-3
- Rosmarinine
Catalog No.:BCN2124
CAS No.:520-65-0
- Spartioidine
Catalog No.:BCN2134
CAS No.:520-59-2
- Spectabiline
Catalog No.:BCN2098
CAS No.:520-55-8
- Psilocin
Catalog No.:BCC6168
CAS No.:520-53-6
- Asebogenin
Catalog No.:BCN7232
CAS No.:520-42-3
- Apigenin
Catalog No.:BCN5658
CAS No.:520-36-5
- Diosmetin
Catalog No.:BCN2356
CAS No.:520-34-3
- Hesperetin
Catalog No.:BCN5657
CAS No.:520-33-2
- Tricin
Catalog No.:BCN5656
CAS No.:520-32-1
- H-Val-pNA
Catalog No.:BCC3139
CAS No.:52084-13-6
- 2-Hydroxy-7-O-methylscillascillin
Catalog No.:BCN5659
CAS No.:52096-50-1
- Mestanolone
Catalog No.:BCC9022
CAS No.:521-11-9
- Dromostanolone propionate
Catalog No.:BCC8954
CAS No.:521-12-0
- Androstenediol
Catalog No.:BCC8828
CAS No.:521-17-5
- Stanolone
Catalog No.:BCC9153
CAS No.:521-18-6
- Bilobetin
Catalog No.:BCN5661
CAS No.:521-32-4
- Sciadopitysin
Catalog No.:BCN5662
CAS No.:521-34-6
- Cannabinol
Catalog No.:BCN7968
CAS No.:521-35-7
- Pedicin
Catalog No.:BCN4845
CAS No.:521-51-7
- Vulpic acid
Catalog No.:BCN6546
CAS No.:521-52-8
- Physcion
Catalog No.:BCN5663
CAS No.:521-61-9
Improvement in gamma-hydroxybutyrate-induced contextual fear memory deficit by systemic administration of NCS-382.[Pubmed:27105320]
Neuroreport. 2016 Jun 15;27(9):627-31.
Low, nonsedative doses of gamma-hydroxybutyric acid (GHB) produce short-term anterograde amnesia in humans and memory impairments in experimental animals. We have previously shown that acute systemic treatment of GHB in adolescent female rats impairs the acquisition, but not the expression, of contextual fear memory while sparing both the acquisition and the expression of auditory cued fear memory. In the brain, GHB binds to specific GHB-binding sites as well as to gamma-aminobutyric acid type B (GABAB) receptors. Although many of the behavioral effects of GHB at high doses have been attributed to its effects on the GABAB receptor, it is unclear which receptor mediates its relatively low-dose memory-impairing effects. The present study examined the ability of the putative GHB receptor antagonist NCS-382 to block the disrupting effects of GHB on fear memory in adolescent rat. Groups of rats received either a single dose of NCS-382 (3-10 mg/kg, intraperitoneally) or vehicle, followed by an injection of either GHB (100 mg/kg, intraperitoneally) or saline. All rats were trained in the fear paradigm, and tested for contextual fear memory and auditory cued fear memory. NCS-382 dose-dependently reversed deficits in the acquisition of contextual fear memory induced by GHB in adolescent rats, with 5 mg/kg of NCS-382 maximally increasing freezing to the context compared with the group administered GHB alone. When animals were tested for cued fear memory, treatment groups did not differ in freezing responses to the tone. These results suggest that low-dose amnesic effects of GHB are mediated by GHB receptors.
In vitro toxicological evaluation of NCS-382, a high-affinity antagonist of gamma-hydroxybutyrate (GHB) binding.[Pubmed:28119166]
Toxicol In Vitro. 2017 Apr;40:196-202.
gamma-Hydroxybutyric acid (GHB), a minor metabolite of the inhibitory neurotransmitter GABA, can accumulate to significant concentrations in the heritable disorder of GABA degradation, succinic semialdehyde dehydrogenase (SSADH) deficiency (SSADHD). Moreover, GHB may be employed in therapeutic settings (treatment of narcolepsy), as well as instances of illicit activity, including acquaintance sexual assault and the induction of euphoria. High-affinity binding sites for GHB in the brain have been identified, although the absolute identity of these receptors remains unclear. Pharmacological antagonism of GHB binding may have multiple instances of therapeutic relevance. The high affinity GHB receptor antagonist, NCS-382 (6,7,8,9-tetrahydro-5-hydroxy-5H-benzo-cyclohept-6-ylideneacetic acid) has not been piloted in humans. To address the potential clinical utility of NCS-382, we have piloted initial studies of its toxicology in HepG2 and primary hepatocyte cells. At high dose (0.5mM), NCS-382 showed no capacity for inhibition of microsomal CYPs (CYP1A2, 2B6, 2C8, 2C9, 2C19, 2D6 and 3A4) and minimal potential for activation of xenobiotic nuclear receptors. Additional cellular integrity and functional assays (viability, oxidative stress, apoptosis, ATP production) revealed little evidence for cytotoxicity, and a low degree of dysregulation of >370 genes actively engaged in the mediation of cellular toxicity. In vitro testing indicates a low probability of cellular toxicity associated with NCS-382.
A pharmacokinetic evaluation and metabolite identification of the GHB receptor antagonist NCS-382 in mouse informs novel therapeutic strategies for the treatment of GHB intoxication.[Pubmed:27891231]
Pharmacol Res Perspect. 2016 Oct 18;4(6):e00265.
Gamma-aminobutyric acid (GABA) is an endogenous inhibitory neurotransmitter and precursor of gamma-hydroxybutyric acid (GHB). NCS-382 (6,7,8,9-tetrahydro-5-hydroxy-5H-benzo-cyclohept-6-ylideneacetic acid), a known GHB receptor antagonist, has shown significant efficacy in a murine model of succinic semialdehyde dehydrogenase deficiency (SSADHD), a heritable neurological disorder featuring chronic elevation of GHB that blocks the final step of GABA degradation. NCS-382 exposures and elimination pathways remain unknown; therefore, the goal of the present work was to obtain in vivo pharmacokinetic data in a murine model and to identify the NCS-382 metabolites formed by mouse and human. NCS-382 single-dose mouse pharmacokinetics were established following an intraperitoneal injection (100, 300, and 500 mg/kg body weight) and metabolite identification was conducted using HPLC-MS/MS. Kinetic enzyme assays employed mouse and human liver microsomes. Upon gaining an understanding of the NCS-382 clearance mechanisms, a chemical inhibitor was used to increase NCS-382 brain exposure in a pharmacokinetic/pharmacodynamic study. Two major metabolic pathways of NCS-382 were identified as dehydrogenation and glucuronidation. The Km for the dehydrogenation pathway was determined in mouse (Km = 29.5 +/- 10.0 mumol/L) and human (Km = 12.7 +/- 4.8 mumol/L) liver microsomes. Comparable parameters for glucuronidation were >100 mumol/L in both species. Inhibition of NCS-382 glucuronidation, in vivo, by diclofenac resulted in increased NCS-382 brain concentrations and protective effects in gamma-butyrolactone-treated mice. These initial evaluations of NCS-382 pharmacokinetics and metabolism inform the development of NCS-382 as a potential therapy for conditions of GHB elevation (including acute intoxication & SSADHD).
Regional Fos-expression induced by gamma-hydroxybutyrate (GHB): comparison with gamma-butyrolactone (GBL) and effects of co-administration of the GABAB antagonist SCH 50911 and putative GHB antagonist NCS-382.[Pubmed:25088910]
Neuroscience. 2014 Sep 26;277:700-15.
gamma-Hydroxybutyrate (GHB) has a complex array of neural actions that include effects on its own high-affinity GHB receptor, the release of neuroactive steroids, and agonist actions at GABAA and GABAB receptors. We previously reported partial overlap in the c-Fos expression patterns produced by GHB and the GABAB agonist, baclofen in rats. The present study extends these earlier findings by examining the extent to which GHB Fos expression and behavioral sedation are prevented by (2S)-(+)-5,5-dimethyl-2-morpholineacetic acid (SCH 50911), a GABAB antagonist, and NCS-382, a putative antagonist at the high-affinity GHB receptor. We also compare Fos expression caused by GHB and its precursor gamma-butyrolactone (GBL), which is a pro-drug for GHB but lacks the high sodium content of the parent GHB molecule. Both GHB (1,000 mg/kg) and GBL (600 mg/kg) induced rapid sedation in rats that lasted over 90 min and caused similar Fos expression patterns, albeit with GBL causing greater activation of the nucleus accumbens (core and shell) and dentate gyrus (granular layer). Pretreatment with SCH 50911 (100mg/kg) partly reversed the sedative effects of GHB and significantly reduced GHB-induced Fos expression in only four regions: the tenia tecta, lateral habenula, dorsal raphe and laterodorsal tegmental nucleus. NCS-382 (50mg/kg) had no effect on GHB-induced sedation or Fos expression. When given alone, both NCS-382 and SCH 50911 increased Fos expression in the bed nucleus of the stria terminalis, central amygdala, parasubthalamic nucleus and nucleus of the solitary tract. SCH 50911 alone affected the Islands of Calleja and the medial, central and paraventricular thalamic nuclei. Overall, this study shows a surprising lack of reversal of GHB-induced Fos expression by two relevant antagonists, both of which have marked intrinsic actions. This may reflect the limited doses tested but also suggests that GHB Fos expression reflects mechanisms independent of GHB and GABAB receptors.
Gammahydroxybutyrate (GHB) receptor ligand effects on evoked synaptic field potentials in CA1 of the rat hippocampal slice.[Pubmed:9503264]
J Neural Transm (Vienna). 1997;104(11-12):1177-93.
GHB produced a concentration-dependent depression of evoked synaptic field potentials (EFPs) recorded extracellularly in the CA1 region of the in vitro rat hippocampal slice. The concentration/response function revealed a threshold near 1 mM, with IC50 of 10.85 mM and a Hill coefficient of 1.29. The gamma-aminobutyric acid B-receptor (GABA-B) agonist baclofen also depressed the EFP, but even maximally effective concentrations of the GABA-B antagonist 2-hydroxy-saclofen (800 microM) could not completely block the GHB-induced EFP depression. Nor was GHB-induced EFP depression blocked by the GHB receptor "antagonist" NCS-382, which does not displace GABA-B receptor ligands. However, NCS-382 produced a concentration-dependent increase in EFP slope. The threshold concentration was about 100 microM but the maximally effective concentration, and thus the IC50, could not be determined in the perfusion slice system. NCS-382 may be an inverse agonist at hippocampal GHB receptors, or else endogenous hippocampal GHB receptor ligands medicate a tonic inhibition in CA1. At concentrations sufficient to induce EFP depression GHB did not alter pH. Although isosmotic sucrose did depress CA1 EFPs it was essentially ineffective at the IC50 for GHB. Gamma-butyrolactone, a prodrug of GHB, was only 1/20th as effective as GHB. This is consistent with previous data suggesting that GBL is freely permeable (does not substantially disturb tonicity) and that brain has very little capacity to either enzymatically convert the lactone to GHB or respond to the lactone itself.
Anti-sedative and anti-cataleptic properties of NCS-382, a gamma-hydroxybutyrate receptor antagonist.[Pubmed:1773824]
Eur J Pharmacol. 1991 Oct 22;203(3):393-7.
NCS-382 possesses antagonistic properties at gamma-hydroxybutyrate receptor sites. Its effect on the sedative/cataleptic behaviour observed in rats after gamma-hydroxybutyrate administration was investigated. NCS-382 diminished, in a dose-dependent manner, the sedative and/or cataleptic effects of gamma-hydroxybutyrate, as revealed by a variety of sensorimotor tests. These results indicate that the well-known sedative/anaesthetic effects induced by gamma-hydroxybutyrate administration are provoked via stimulation of a specific class(es) of gamma-hydroxybutyrate receptors which exist in the rat brain and which could mediate a local stimulation of opiate synthesis and release.
A specific gamma-hydroxybutyrate receptor ligand possesses both antagonistic and anticonvulsant properties.[Pubmed:2173754]
J Pharmacol Exp Ther. 1990 Nov;255(2):657-63.
Administration of gamma-hydroxybutyrate (GHB) to animals induces electroencephalographic and behavioral changes that resemble petit-mal seizures. Furthermore, these GHB-induced electroencephalogram-behavioral changes can be blocked by anticonvulsant drugs, which are specific in their action against petit-mal seizures. These effects of GHB on electroencephalogram and behavior may well be due to an effect of exogenously administrated GHB on GHB-mediated systems in the brain. GHB has many properties of a neuromodulator including the existence of receptors with a specific affinity for this compound. A synthetic structural analog of GHB, NCS-382, possessed anticonvulsant activity against several animal models of seizure and, in particular, against that induced by GHB administration. NCS-382 was also shown to be an antagonist at GHB receptor sites and blocked the neuropharmacologic effects induced in the striatum and hippocampus by GHB administration. In particular, NCS-382 inhibited the increase in cGMP levels and in inositol phosphate turnover induced by GHB in hippocampus. Furthermore, in vivo dialysis demonstrated that NCS-382 blocked the increased release of dopamine in striatum after GHB administration in vivo. Thus, this ligand appears to be the first described antagonist substance for GHB receptor(s). These results suggest that NCS-382 may represent a harbinger for a new class of anticonvulsant drugs that most probably act by modifying the endogenous GHB system.


