Fosamprenavir Calcium SaltProdrug of antiretroviral protease inhibitor amprenavir CAS# 226700-81-8 |
- Saquinavir
Catalog No.:BCC1921
CAS No.:127779-20-8
- Saquinavir mesylate
Catalog No.:BCC1922
CAS No.:149845-06-7
- Nelfinavir Mesylate
Catalog No.:BCC1794
CAS No.:159989-65-8
- Lopinavir
Catalog No.:BCC3621
CAS No.:192725-17-0
- Darunavir
Catalog No.:BCC3623
CAS No.:206361-99-1
- BMS-626529
Catalog No.:BCC1427
CAS No.:701213-36-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 226700-81-8 | SDF | Download SDF |
| PubChem ID | 131535 | Appearance | Powder |
| Formula | C25H34CaN3O9PS | M.Wt | 623.67 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | GW433908G | ||
| Solubility | DMSO : 1.8 mg/mL (2.89 mM; Need ultrasonic and warming) H2O : 0.25 mg/mL (0.40 mM; Need ultrasonic and warming) | ||
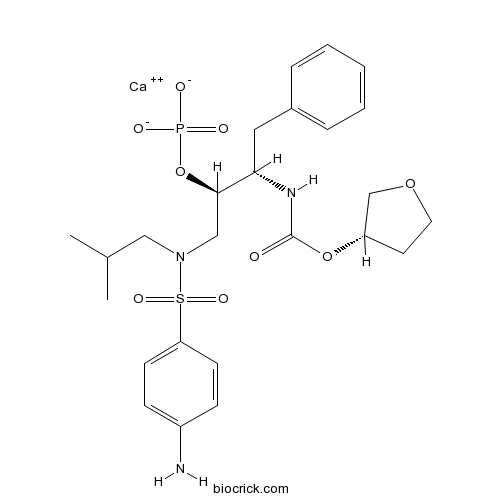
| Chemical Name | calcium;[(2R,3S)-1-[(4-aminophenyl)sulfonyl-(2-methylpropyl)amino]-3-[[(3S)-oxolan-3-yl]oxycarbonylamino]-4-phenylbutan-2-yl] phosphate | ||
| SMILES | CC(C)CN(CC(C(CC1=CC=CC=C1)NC(=O)OC2CCOC2)OP(=O)([O-])[O-])S(=O)(=O)C3=CC=C(C=C3)N.[Ca+2] | ||
| Standard InChIKey | PMDQGYMGQKTCSX-HQROKSDRSA-L | ||
| Standard InChI | InChI=1S/C25H36N3O9PS.Ca/c1-18(2)15-28(39(33,34)22-10-8-20(26)9-11-22)16-24(37-38(30,31)32)23(14-19-6-4-3-5-7-19)27-25(29)36-21-12-13-35-17-21;/h3-11,18,21,23-24H,12-17,26H2,1-2H3,(H,27,29)(H2,30,31,32);/q;+2/p-2/t21-,23-,24+;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | GW433908 is a phosphate ester prodrug of the antiretroviral protease inhibitor amprenavir, with improved solubility over the parent molecule and a potential for reduced pill burden on current dosing regimens; GW433908G is the calcium salt of the prodrug.
IC50 Value:
Target: HIV Protease
in vitro: There were no significant changes in buprenorphine or PI plasma levels and no significant changes in medication adverse effects or opioid withdrawal. Increased concentrations of the inactive metabolite buprenorphine-3-glucuronide suggested that darunavir-ritonavir and fosamprenavir-ritonavir induced glucuronidation of buprenorphine[1].
in vivo: Fosamprenavir-ritonavir administered with methadone did not alter plasma amprenavir pharmacokinetics compared with historical control data; nor did it alter the unbound R-methadone at 2 and 6 hours after methadone dosing. Pharmacodynamic indexes remained essentially unchanged after adding fosamprenavir-ritonavir to methadone [2]. After a high-fat meal compared with fasting, (1) the bioavailability of GW433908G suspension was decreased by 20% and Cmax by 41%, and (2) for GW433908G tablets, there was no influence on AUC(12% lower Cmax). After a low-fat meal compared with fasting, (1) there was bioequivalence for GW433908G tablets, but (2) bioavailability was decreased by 23% for amprenavir capsules (Cmax was also lower, by 46%) [3].
Clinical trial: Study of an Investigational Regimen Including FDA Approved HIV Drugs In HIV-Infected Pediatric Subjects. Phase 2 References: | |||||

Fosamprenavir Calcium Salt Dilution Calculator

Fosamprenavir Calcium Salt Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6034 mL | 8.0171 mL | 16.0341 mL | 32.0682 mL | 40.0853 mL |
| 5 mM | 0.3207 mL | 1.6034 mL | 3.2068 mL | 6.4136 mL | 8.0171 mL |
| 10 mM | 0.1603 mL | 0.8017 mL | 1.6034 mL | 3.2068 mL | 4.0085 mL |
| 50 mM | 0.0321 mL | 0.1603 mL | 0.3207 mL | 0.6414 mL | 0.8017 mL |
| 100 mM | 0.016 mL | 0.0802 mL | 0.1603 mL | 0.3207 mL | 0.4009 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GW433908G is a selective inhibitor of antiretroviral protease with the concentration of 1395 mg nM once daily in clinical trial [1].
Antiretroviral protease is a subfamily of protease inhibitors and plays a pivotal role in treating HIV/AIDS and HCV infection. It has been reported that drugs designed as protease inhibitors can prevent viral replication by selectively binding to viral proteases (e.g. HIV-1 protease) and blocking proteolytic cleavage of protein precursors that are necessary for the production of infectious viral particles [2] [3].
GW433908 is a potent antiretroviral protease inhibitor and has improved solubility than amprenavir capsules. Many clinical studies have been done to examine GE433908 safety and pharmacokinetic profiles. In healthy male volunteers, administered GW433908 as tablets or suspension, food had slight effect on its pharmacokinetics and with fewer tablets GW433908 were well tolerated and delivered plasma amprenavir concentrations equivalent to the recommended therapeutic amprenavir dose which may be of clinical benefit in the treatment of HIV infection [4]. As a phosphate ester prodrug of the antiretroviral protease inhibitor amprenavir, GW433908 (1395 mg, QD) combined with efavirenz (200 mg, QD) decreased plasma APV exposure when tested with healthy volunteers [1].
References:
[1]. Wire, M.B., et al., Pharmacokinetics and safety of GW433908 and ritonavir, with and without efavirenz, in healthy volunteers. Aids, 2004. 18(6): p. 897-907.
[2]. Hamada, Y., et al., High incidence of renal stones among HIV-infected patients on ritonavir-boosted atazanavir than in those receiving other protease inhibitor-containing antiretroviral therapy. Clin Infect Dis, 2012. 55(9): p. 1262-9.
[3]. Zheng, Y., et al., Antiretroviral therapy and efficacy after virologic failure on first-line boosted protease inhibitor regimens. Clin Infect Dis, 2014. 59(6): p. 888-96.
[4]. Falcoz, C., et al., Pharmacokinetics of GW433908, a prodrug of amprenavir, in healthy male volunteers. J Clin Pharmacol, 2002. 42(8): p. 887-98.
- 3-Hydroxy-12-oleanene-23,28-dioic acid
Catalog No.:BCN1482
CAS No.:226562-47-6
- Amaronol B
Catalog No.:BCN5071
CAS No.:226561-02-0
- Amaronol A
Catalog No.:BCN5070
CAS No.:226560-96-9
- 4,6,7-Trihydroxycoumarin
Catalog No.:BCC9203
CAS No.:22649-24-7
- Torachrysone
Catalog No.:BCN5069
CAS No.:22649-04-3
- Cinacalcet
Catalog No.:BCC1483
CAS No.:226256-56-0
- Methyl kulonate
Catalog No.:BCN7952
CAS No.:22611-37-6
- Kulactone
Catalog No.:BCN7953
CAS No.:22611-36-5
- Demethoxycurcumin
Catalog No.:BCN5974
CAS No.:22608-11-3
- Auriculine
Catalog No.:BCN2013
CAS No.:22595-00-2
- Lucidioline
Catalog No.:BCN7413
CAS No.:22594-91-8
- (+)-Catechin hydrate
Catalog No.:BCN2309
CAS No.:225937-10-0
- Bakkenolide Db
Catalog No.:BCN7117
CAS No.:226711-23-5
- 2-(3,4-dihydroxyphenyl)-2-hydroxypropanoic acid
Catalog No.:BCN6296
CAS No.:22681-72-7
- NPS 2390
Catalog No.:BCC6119
CAS No.:226878-01-9
- Kaempferol-3-beta-O-glucuronide
Catalog No.:BCN2503
CAS No.:22688-78-4
- Quercetin-3-O-glucuronide
Catalog No.:BCN3149
CAS No.:22688-79-5
- Lansoprazole sodium
Catalog No.:BCC4298
CAS No.:226904-00-3
- 4-Hydroxy-1,10-secocadin-5-ene-1,10-dione
Catalog No.:BCN6661
CAS No.:226904-40-1
- LB42708
Catalog No.:BCC5344
CAS No.:226929-39-1
- Emapunil
Catalog No.:BCC5521
CAS No.:226954-04-7
- 3,6-Dimethoxyapigenin
Catalog No.:BCN4830
CAS No.:22697-65-0
- Hyponine E
Catalog No.:BCC8999
CAS No.:226975-99-1
- 9-Deacetyl-9-benzoyl-10-debenzoyl-4beta,20-epoxytaxchinin A
Catalog No.:BCN7676
CAS No.:227011-48-5
Gateways to clinical trials.[Pubmed:19536362]
Methods Find Exp Clin Pharmacol. 2009 Apr;31(3):183-226.
(+)-Dapoxetine hydrochloride, [(123)I]-BZA, 9-Aminocamptothecin; Abacavir sulfate/lamivudine, Adalimumab, Adefovir dipivoxil, Alemtuzumab, Alvocidib hydrochloride, Ambrisentan, Amsilarotene, Anacetrapib, Anakinra, Apricitabine, Aripiprazole, Arsenic trioxide, Atazanavir sulfate, Atazanavir/ritonavir, Atrasentan, Azacitidine; Banoxantrone, Bazedoxifene acetate, Bevacizumab, Bexarotene, Biphasic insulin aspart, Bortezomib, Bosentan, Bromfenac; Cachectin, Calcipotriol/betamethasone dipropionate, Canakinumab, Carfilzomib, CAT-354, CCX-282, Certolizumab pegol, Cetuximab, Choline fenofibrate, Clevudine, Clofarabine, CNTO-328, Corifollitropin alfa, Crofelemer; Daptomycin, Darbepoetin alfa, Darunavir, Dasatinib, Decitabine, Deferasirox, Denosumab, Duloxetine hydrochloride, Dutasteride; Emtricitabine, Enfuvirtide, Entecavir, Epoetin zeta, Erlotinib hydrochloride, Escitalopram oxalate, Eslicarbazepine acetate, Eszopiclone, Etravirine, Everolimus, Exenatide, Ezetimibe, Ezetimibe/simvastatin; Farglitazar, Febuxostat, Fosamprenavir calcium, FX-06; Gabapentin enacarbil, Gefitinib; HIVIS DNA; Imatinib mesylate, INCB- 18424, Indacaterol, Inotuzumab ozogamicin, Insulin detemir; JNJ-26854165; Lacosamide, Landiolol, Laromustine, Lenalidomide, Liposomal doxorubicin, L-NAME, Lopinavir, Lopinavir/ritonavir, Lumiracoxib; Maraviroc, Mepolizumab, Methoxy polyethylene glycol- epoetin-beta, Miglustat, MK-0493, MVA-CMDR, Mycophenolic acid sodium salt; Natalizumab, Nepafenac, Neratinib, Neridronic acid, Nesiritide, Nilotinib hydrochloride monohydrate; Olmesartan medoxomil, Omacetaxine mepesuccinate, Omalizumab; Paclitaxel poliglumex, Palifermin, Patupilone, Pegfilgrastim, Peginterferon alfa-2a, Peginterferon alfa-2b, Peginterferon alfa-2b/ ribavirin, Pemetrexed disodium, PHA-848125, Pitavastatin calcium, Posaconazole, Povidone-iodine liposome complex, Prasugrel, Pregabalin, Prucalopride; Raltegravir potassium, Retigabine, Revaprazan hydrochloride, rhFSH, Rilpivirine, Rivaroxaban, Romidepsin, Rosuvastatin calcium, RWJ-676070; SAR-109659, Sitagliptin phosphate monohydrate, Sorafenib, Stavudine/Lamivudine/Nevirapine, Sunitinib malate; Tadalafil, Telaprevir, Telbivudine, Tenofovir disoproxil fumarate, Tenofovir disoproxil fumarate/emtricitabine, Tenofovir disoproxil fumarate/emtricitabine/efavirenz, Teriparatide, Tigecycline, Tiotropium bromide, Tipifarnib, Tipranavir, Tocilizumab, Trifluridine/TPI; UP-780; Vandetanib, Vardenafil hydrochloride hydrate, Vatalanib succinate, Vitespen, Vorinostat; Yttrium 90 (90Y) ibritumomab tiuxetan; Zoledronic acid monohydrate.
Gateways to clinical trials.[Pubmed:19557204]
Methods Find Exp Clin Pharmacol. 2009 May;31(4):263-98.
(-)-Gossypol; Abacavir sulfate/lamivudine, ACAM-1000, ACE-011, Agomelatine, AGS-004, Alemtuzumab, Alvocidib hydrochloride, AMG-317, Amlodipine, Aripiprazole, Atazanavir sulfate, Azacitidine; Becatecarin, Belinostat, Bevacizumab, BMS-387032, BMS-690514, Bortezomib; Casopitant mesylate, Cetuximab, Choline fenofibrate, CK-1827452, Clofarabine, Conivaptan hydrochloride; Dabigatran etexilate, DADMe-Immucillin-H, Darbepoetin alfa, Darunavir, Dasatinib, DC-WT1, Decitabine, Deferasirox, Degarelix acetate, Denenicokin, Denosumab, Dienogest, Duloxetine hydrochloride; Ecogramostim, Eculizumab, Edoxaban tosilate, Elacytarabine, Elesclomol, Eltrombopag olamine, Enfuvirtide, Enzastaurin hydrochloride, Eribulin mesilate, Erlotinib hydrochloride, Escitalopram oxalate, Eszopiclone, Etravirine; Flibanserin, Fludarabine, Fondaparinux sodium, Fosamprenavir calcium; Gefitinib, Genistein; I-131-L19-SIP, Idrabiotaparinux sodium, Imatinib mesylate, IMGN-901, Ipilimumab; Laromustine, Lenalidomide, Liposomal cisplatin, Liraglutide, Lisdexamfetamine mesilate, Lopinavir, Lopinavir/ritonavir; Maraviroc, MDV-3100, Mecasermin rinfabate, MP-470, Mycophenolic acid sodium salt; Naproxcinod, NB-002, Nesiritide, Nilotinib hydrochloride monohydrate, NK-012; Palonosetron hydrochloride, Panobinostat, Pegfilgrastim, Peginterferon alfa-2a, Pitavastatin calcium, PL-3994, Plerixafor hydrochloride, Plitidepsin, PM-10450; Raltegravir potassium, Recombinant human soluble thrombomodulin, ReoT3D, RHAMM R3 peptide, Rivaroxaban, Romiplostim, Rosuvastatin calcium, Rozrolimupab; Sabarubicin hydrochloride, Salinosporamide A, Sirolimus-eluting stent, Smallpox (Vaccinia) Vaccine, Live, Sorafenib; Tenofovir disoproxil fumarate, Tenofovir disoproxil fumarate/emtricitabine, Teriparatide, Tipifarnib, Tipranavir, Trabectedin, Trifluridine/TPI; Vardenafil hydrochloride hydrate, Vinflunine, Volociximab, Vorinostat; Ximelagatran; Yttrium 90 (90Y) ibritumomab tiuxetan; Ziprasidone hydrochloride, Zoledronic acid monohydrate.
Gateways to clinical trials.[Pubmed:15148527]
Methods Find Exp Clin Pharmacol. 2004 Apr;26(3):211-44.
Gateways to Clinical Trials is a guide to the most recent clinical trials in current literature and congresses. The data in the following tables has been retrieved from the Clinical Studies Knowledge Area of Prous Science Integrity(R), the drug discovery and development portal, http://integrity.prous.com. This issue focuses on the following selection of drugs: ABI-007, adalimumab, adefovir dipivoxil, alefacept, alemtuzumab, 3-AP, AP-12009, APC-8015, L-Arginine hydrochloride, aripiprazole, arundic acid, avasimibe; Bevacizumab, bivatuzumab, BMS-181176, BMS-184476, BMS-188797, bortezomib, bosentan, botulinum toxin type B, BQ-123, BRL-55730, bryostatin 1; CEP-1347, cetuximab, cinacalcet hydrochloride, CP-461, CpG-7909; D-003, dabuzalgron hydrochloride, darbepoetin alfa, desloratadine, desoxyepothilone B, dexmethylphenidate hydrochloride, DHA-paclitaxel, diflomotecan, DN-101, DP-b99, drotrecogin alfa (activated), duloxetine hydrochloride, duramycin; Eculizumab, Efalizumab, EKB-569, elcometrine, enfuvirtide, eplerenone, erlotinib hydrochloride, ertapenem sodium, eszopiclone, everolimus, exatecan mesilate, ezetimibe; Fenretinide, fosamprenavir calcium, frovatriptan; GD2L-KLH conjugate vaccine, gefitinib, glufosfamide, GTI-2040; Hexyl insulin M2, human insulin, hydroquinone, gamma-Hydroxybutyrate sodium; IL-4(38-37)-PE38KDEL, imatinib mesylate, indisulam, inhaled insulin, ixabepilone; KRN-5500; LY-544344; MDX-210, melatonin, mepolizumab, motexafin gadolinium; Natalizumab, NSC-330507, NSC-683864; 1-Octanol, omalizumab, ortataxel; Pagoclone, peginterferon alfa-2a, peginterferon alfa-2b, pemetrexed disodium, phenoxodiol, pimecrolimus, plevitrexed, polyphenon E, pramlintide acetate, prasterone, pregabalin, PX-12; QS-21; Ragaglitazar, ranelic acid distrontium salt, RDP-58, recombinant glucagon-like peptide-1 (7-36) amide, repinotan hydrochloride, rhEndostatin, rh-Lactoferrin, (R)-roscovitine; S-8184, semaxanib, sitafloxacin hydrate, sitaxsentan sodium, sorafenib, synthadotin; Tadalafil, tesmilifene hydrochloride, theratope, tipifarnib, tirapazamine, topixantrone hydrochloride, trabectedin, traxoprodil, Tri-Luma; Valdecoxib, valganciclovir hydrochloride, vinflunine; Ximelagatran; Ziconotide.
Gateways to clinical trials.[Pubmed:16810345]
Methods Find Exp Clin Pharmacol. 2006 Apr;28(3):185-206.
Gateways to Clinical Trials are a guide to the most recent clinical trials in current literature and congresses. The data in the following tables have been retrieved from the Clinical Trials Knowledge Area of Prous Science Integrity, the drug discovery and development portal, http://integrity.prous.com. This issue focuses on the following selection of drugs: ABT-510, adalimumab, alefacept, alemtuzumab, AMG-531, anakinra, armodafinil, asenapine maleate, atazanavir sulfate, atorvastatin; Bortezomib, bosentan; CEB-1555, cetuximab, ciclesonide, clodronate, CT-011; Darifenacin hydrobromide, desloratadine; E-7010, ecallantide, eculizumab, efalizumab, eltrombopag, erlotinib hydrochloride, eslicarbazepine acetate, eszopiclone, ezetimibe; Febuxostat, fosamprenavir calcium, fulvestrant; Gefitinib, genistein; Haemophilus influenzae B vaccine, human papillomavirus vaccine; Imatinib mesylate, insulin glargine; Lenalidomide, liposomal cisplatin; MAb G250, mapatumumab, midostaurin, MP4, mycophenolic acid sodium salt; Natalizumab, neridronic acid, NSC-330507; Oblimersen sodium, ofatumumab, omalizumab, oral insulin, oregovomab; Paliperidone, parathyroid hormone (human recombinant), peginterferon alfa-2a, peginterferon alfa-2b, peginterferon alfa-2b/ribavirin, pegylated arginine deiminase 20000, pemetrexed disodium, pimecrolimus, pitavastatin, pneumococcal 7-valent conjugate vaccine, prasterone, pregabalin, pumosetrag hydrochloride; Recombinant malaria vaccine, retigabine, rivaroxaban, Ro-26-9228, romidepsin, rosuvastatin calcium, rotavirus vaccine; SGN-30, sitaxsentan sodium, solifenacin succinate, sorafenib, sunitinib malate; Tadalafil, tegaserod maleate, temsirolimus, TER-199, tifacogin, tiludronic acid, tiotropium bromide; Vildagliptin, VNP-40101M, vorinostat; YM-150, yttrium 90 (90Y) ibritumomab tiuxetan; Zanolimumab, zoledronic acid monohydrate.
Gateways to clinical trials.[Pubmed:15538546]
Methods Find Exp Clin Pharmacol. 2004 Sep;26(7):587-612.
Gateways to Clinical Trials is a guide to the most recent clinical trials in current literature and congresses. The data in the following tables has been retrieved from the Clinical Trials Knowledge Area of Prous Science Integrity, the drug discovery and development portal, http://integrity.prous.com. This issue focuses on the following selection of drugs: 101M, 166Ho-DOTMP, 3-AP; Abatacept, abetimus sodium, ACR-16, adefovir dipivoxil, alefacept, AMD-070, aminolevulinic acid hexyl ester, anatumomab mafenatox, anti-CTLA-4 MAb, antigastrin therapeutic vaccine, AP-12009, AP-23573, APC-8024, aripiprazole, ATL-962, atomoxetine hydrochloride; Bevacizumab, bimatoprost, bortezomib, bosentan, BR-1; Calcipotriol/betamethasone dipropionate, cinacalcet hydrochloride, clofazimine, colchicine, cold-adapted influenza vaccine trivalent, CRM197; Desloratadine, desoxyepothilone B, diethylhomospermine; Edodekin alfa, efalizumab, elcometrine, eletriptan, enfuvirtide, entecavir, EP-2101, eplerenone, erlotinib hydrochloride, etoricoxib, everolimus, exherin, ezetimibe; Febuxostat, fluorescein lisicol, fosamprenavir calcium, frovatriptan; Hemoglobin raffimer, HSPPC-96, human insulin; Imatinib mesylate, insulin detemir, insulin glargine, IRX-2, istradefylline, IV gamma-globulin, ixabepilone; Kahalalide F; L-759274, levodopa/carbidopa/entacapone, licofelone, lonafarnib, lopinavir, lurtotecan, LY-156735; MAb G250, mecasermin, melatonin, midostaurin, muraglitazar; Nesiritide, nitronaproxen; O6-Benzylguanine, olmesartan medoxomil, olmesartan medoxomil/hydrochlorothiazide, omapatrilat, oral insulin; Parecoxib sodium, PCK-3145, peginterferon alfa-2a, peginterferon alfa-2b, peginterferon alfa-2b/ ribavirin, pemetrexed disodium, peptide YY3-36, PG-CPT, phenoxodiol, pimecrolimus, posaconazole; Rasagiline mesilate, rDNA insulin, RG228, rimonabant hydrochloride, rosuvastatin calcium, rotigotine hydrochloride; S-3304, safinamide mesilate, salcaprozic acid sodium salt, SDZ-SID-791, SGN-30, soblidotin, squalamine; Telmisartan/hydrochlorothiazide, testosterone gel, TF(c)-KLH conjugate vaccine, TH-9507, theraloc, tipifarnib, tocilizumab, travoprost; ValboroPro, valdecoxib, veglin, voriconazole; Ximelagatran.


