CinacalcetCAS# 226256-56-0 |
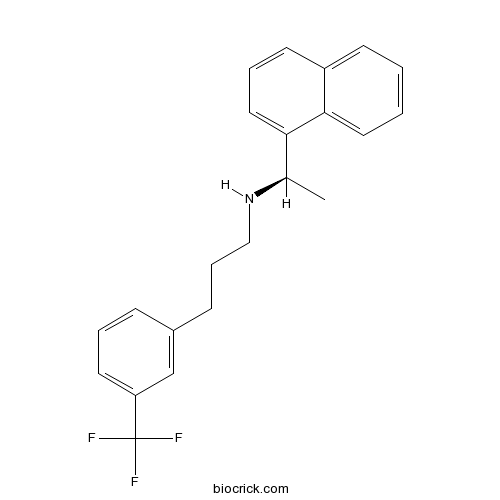
- Cinacalcet HCl
Catalog No.:BCC4408
CAS No.:364782-34-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 226256-56-0 | SDF | Download SDF |
| PubChem ID | 156419 | Appearance | Powder |
| Formula | C22H22F3N | M.Wt | 357.41 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | AMG 073 | ||
| Solubility | DMSO : ≥ 100 mg/mL (279.79 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | N-[(1R)-1-naphthalen-1-ylethyl]-3-[3-(trifluoromethyl)phenyl]propan-1-amine | ||
| SMILES | CC(C1=CC=CC2=CC=CC=C21)NCCCC3=CC(=CC=C3)C(F)(F)F | ||
| Standard InChIKey | VDHAWDNDOKGFTD-MRXNPFEDSA-N | ||
| Standard InChI | InChI=1S/C22H22F3N/c1-16(20-13-5-10-18-9-2-3-12-21(18)20)26-14-6-8-17-7-4-11-19(15-17)22(23,24)25/h2-5,7,9-13,15-16,26H,6,8,14H2,1H3/t16-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cinacalcet is an orally active, allosteric agonist of Ca receptor (CaR), used for cardiovascular disease treatment.In Vivo:Cinacalcet HCl (5 and 10 mg/kg) results in a significant reduction in parathyroid gland weight in 5/6 nephrectomy animals. In sham animals, Cinacalcet HCl has no effect on parathyroid gland cell proliferation or parathyroid weight compared with vehicle treatment. There are no differences in serum phosphorus levels in Cinacalcet HCl (10, 5, or 1 mg/kg) treated 5/6 nephrectomized animals compared with vehicle-treated 5/6 nephrectomized animals. Cinacalcet HCl treatment significantly reduces blood ionized calcium levels in sham animals[1]. Cinacalcet (30 mg/kg/24 h) leads to a marked reduction in circulating parathyroid hormone and a modest reduction in serum Ca. Cinacalcet does not alter UCa when the GHS rats are fed the normal Ca diet but lowers UCa when they are fed the low Ca diet. Cinacalcet does not alter U supersaturation with respect to either CaOx or CaHPO4 on either diet[2]. References: | |||||

Cinacalcet Dilution Calculator

Cinacalcet Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7979 mL | 13.9895 mL | 27.9791 mL | 55.9581 mL | 69.9477 mL |
| 5 mM | 0.5596 mL | 2.7979 mL | 5.5958 mL | 11.1916 mL | 13.9895 mL |
| 10 mM | 0.2798 mL | 1.399 mL | 2.7979 mL | 5.5958 mL | 6.9948 mL |
| 50 mM | 0.056 mL | 0.2798 mL | 0.5596 mL | 1.1192 mL | 1.399 mL |
| 100 mM | 0.028 mL | 0.1399 mL | 0.2798 mL | 0.5596 mL | 0.6995 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Cinacalcet(AMG-073) is an orally active, second-generation calcimimetic compound; AMG 073 represents a new class of compounds for the treatment of hyperparathyroidism.
- Methyl kulonate
Catalog No.:BCN7952
CAS No.:22611-37-6
- Kulactone
Catalog No.:BCN7953
CAS No.:22611-36-5
- Demethoxycurcumin
Catalog No.:BCN5974
CAS No.:22608-11-3
- Auriculine
Catalog No.:BCN2013
CAS No.:22595-00-2
- Lucidioline
Catalog No.:BCN7413
CAS No.:22594-91-8
- (+)-Catechin hydrate
Catalog No.:BCN2309
CAS No.:225937-10-0
- Cyclothiazide
Catalog No.:BCC6759
CAS No.:2259-96-3
- Epifriedelanol acetate
Catalog No.:BCN5068
CAS No.:2259-07-6
- PAR 4 (1-6)
Catalog No.:BCC3956
CAS No.:225779-44-2
- Symphytine
Catalog No.:BCN1975
CAS No.:22571-95-5
- Zeorin
Catalog No.:BCN5067
CAS No.:22570-53-2
- Bisabolol Oxide A
Catalog No.:BCC8133
CAS No.:22567-36-8
- Torachrysone
Catalog No.:BCN5069
CAS No.:22649-04-3
- 4,6,7-Trihydroxycoumarin
Catalog No.:BCC9203
CAS No.:22649-24-7
- Amaronol A
Catalog No.:BCN5070
CAS No.:226560-96-9
- Amaronol B
Catalog No.:BCN5071
CAS No.:226561-02-0
- 3-Hydroxy-12-oleanene-23,28-dioic acid
Catalog No.:BCN1482
CAS No.:226562-47-6
- Fosamprenavir Calcium Salt
Catalog No.:BCC1581
CAS No.:226700-81-8
- Bakkenolide Db
Catalog No.:BCN7117
CAS No.:226711-23-5
- 2-(3,4-dihydroxyphenyl)-2-hydroxypropanoic acid
Catalog No.:BCN6296
CAS No.:22681-72-7
- NPS 2390
Catalog No.:BCC6119
CAS No.:226878-01-9
- Kaempferol-3-beta-O-glucuronide
Catalog No.:BCN2503
CAS No.:22688-78-4
- Quercetin-3-O-glucuronide
Catalog No.:BCN3149
CAS No.:22688-79-5
- Lansoprazole sodium
Catalog No.:BCC4298
CAS No.:226904-00-3
Efficacy of low-dose cinacalcet on alternate days for the treatment of secondary hyperparathyroidism in hemodialysis patients: a single-center study.[Pubmed:28223837]
Int J Nephrol Renovasc Dis. 2017 Feb 7;10:47-53.
INTRODUCTION: Cinacalcet is effective in reducing serum parathyroid hormone (PTH) in patients with secondary hyperparathyroidism (HPT). This study focused on testing whether a prescription of low-dose Cinacalcet on alternate days could be an option for treatment of secondary HPT. MATERIALS AND METHODS: A retrospective clinical study was conducted on chronic maintenance hemodialysis patients. Patients with secondary HPT who received Cinacalcet at a starting dose of 25 mg on alternate days were reviewed (low-dose group). Patients who were being treated with a standard dose of Cinacalcet in the same period of time were selected as the control group. The primary outcome was difference in the percentage of patients achieving >30% reduction of intact parathyroid hormone (iPTH) levels at 16 weeks. The changes of serum iPTH and other biochemical data were also tested. RESULTS: A total of 30 patients (16 low doses and 14 controls) took part in the study. Baseline iPTH levels in the low-dose and control group were 1,065.9+/-477.7 and 1,214.1+/-497.6 pg/mL, respectively (p=0.413). The analysis showed that the percentage of patients who achieved the primary outcome showed little or no difference (33.3% in the low-dose group compared with 38.5% in the control group, p=1.0). Serum iPTH reduction during 16 weeks of study period in the low-dose and control group was 253.5+/-316.1 and 243.4+/-561.3 pg/mL, respectively (p=0.957). There was no difference in the adverse events between both groups. CONCLUSION: Among patients with secondary HPT, initial treatment with Cinacalcet 25 mg on alternate days can decrease serum PTH levels. The role of low-dose Cinacalcet in secondary HPT should be further determined in large-scale, randomized controlled trials.
Cinacalcet in peritoneal dialysis patients: one-center experience.[Pubmed:28355398]
J Bras Nefrol. 2017 Mar;39(1):42-45.
INTRODUCTION: Secondary hyperparathyroidism is the target of several therapeutic strategies, including the use of Cinacalcet. Most studies were done only in hemodialysis patients, with few data from peritoneal dialysis patients. OBJECTIVE: The aim of our work was to evaluate the effectiveness of Cinacalcet in secondary hyperparathyroidism in a one-center peritoneal dialysis patients. METHODS: A retrospective study was performed in 27 peritoneal dialysis patients with moderate to severe secondary hyperparathyroidism (PTHi > 500 pg/mL with normal or elevated serum calcium levels) treated with Cinacalcet. Demographic, clinical and laboratory parameters at the beginning of Cinacalcet therapy, second, fourth, sixth months after and at the time it was finished were analyzed. RESULTS: Patients were under peritoneal dialysis at 30.99 +/- 16.58 months and were treated with Cinacalcet for 15.6 +/- 13.4 months; 21 (77.8%) patients showed adverse gastrointestinal effects; PTHi levels at the beginning of Cinacalcet therapy were 1145 +/- 449 pg/mL. The last PTHi levels under Cinacalcet therapy was 1131 +/- 642 pg/mL. PTHi reduction was statistically significant at 2 months after the beginning of Cinacalcet (p = 0.007) but not in the following evaluations. CONCLUSION: It is necessary the development of new forms of Cinacalcet presentation, in order to avoid gastrointestinal effects adverse factors and to improve therapeutic adherence.


