DemethoxycurcuminCAS# 22608-11-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 22608-11-3 | SDF | Download SDF |
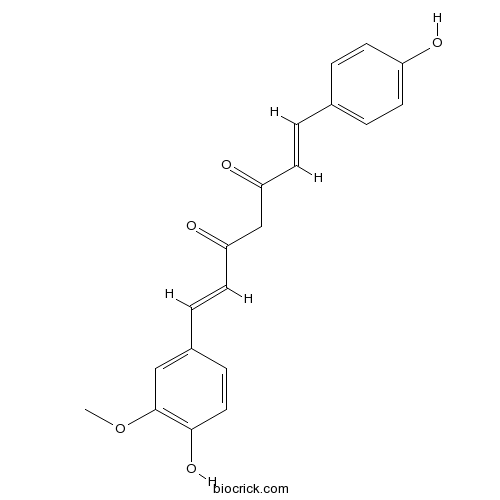
| PubChem ID | 5469424 | Appearance | Yellow-orange powder |
| Formula | C20H18O5 | M.Wt | 338.35 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Synonyms | Curcumin II; Desmethoxycurcumin; Monodemethoxycurcumin;33171-16-3;24939-17-1 | ||
| Solubility | DMSO : 100 mg/mL (295.55 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | (1E,6E)-1-(4-hydroxy-3-methoxyphenyl)-7-(4-hydroxyphenyl)hepta-1,6-diene-3,5-dione | ||
| SMILES | COC1=C(C=CC(=C1)C=CC(=O)CC(=O)C=CC2=CC=C(C=C2)O)O | ||
| Standard InChIKey | HJTVQHVGMGKONQ-LUZURFALSA-N | ||
| Standard InChI | InChI=1S/C20H18O5/c1-25-20-12-15(6-11-19(20)24)5-10-18(23)13-17(22)9-4-14-2-7-16(21)8-3-14/h2-12,21,24H,13H2,1H3/b9-4+,10-5+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Demethoxycurcumin is a potential additive natural product in combination with chemotherapeutic agents in drug-resistant cancers, which has anti-acanthamoebic, anti-proliferative, antimetastatic, anti-inflammatory, antioxidant activities. It inhibited P-glycoprotein-mediated ATP hydrolysis under concentrations of <1 μM and efficiently inhibited 200 μM verapamil-stimulated ATPase activity. |
| Targets | MMP(e.g.TIMP) | COX | TNF-α | NF-kB | EGFR | HSP (e.g. HSP90) | AMPK | ATPase | IL Receptor |
| In vitro | Demethoxycurcumin inhibits energy metabolic and oncogenic signaling pathways through AMPK activation in triple-negative breast cancer cells.[Pubmed: 23777448]J Agric Food Chem. 2013 Jul 3;61(26):6366-75.Demethoxycurcumin (DMC), curcumin (Cur), and bisDemethoxycurcumin (BDMC) are major forms of curcuminoids found in the rhizomes of turmeric.
Demethoxycurcumin modulates prostate cancer cell proliferation via AMPK-induced down-regulation of HSP70 and EGFR.[Pubmed: 22849866]J Agric Food Chem. 2012 Aug 29;60(34):8427-34.Curcumin (Cur), Demethoxycurcumin (DMC), and bisDemethoxycurcumin (BDMC) are major forms of curcuminoids found in the rhizomes of turmeric.
|
| Kinase Assay | Demethoxycurcumin modulates human P-glycoprotein function via uncompetitive inhibition of ATPase hydrolysis activity.[Pubmed: 25594233]J Agric Food Chem. 2015 Jan 28;63(3):847-55.Curcuminoids are major components of Curcuma longa L., which is widely used as spice in food. This study aimed at identifying whether curcumin, Demethoxycurcumin, and bisDemethoxycurcumin could modulate efflux function of human P-glycoprotein and be used as chemosensitizers in cancer treatments.
|
| Cell Research | Anti-Acanthamoebic properties of resveratrol and demethoxycurcumin.[Pubmed: 23010569]Exp Parasitol. 2012 Dec;132(4):519-23.Acanthamoeba is an opportunist protist pathogen that is known to infect the cornea to produce eye keratitis and the central nervous system to produce fatal granulomatous encephalitis. Early diagnosis, followed by aggressive treatment using a combination of drugs is a prerequisite in successful treatment but even then, prognosis remains poor due to lack of effective drugs.
|

Demethoxycurcumin Dilution Calculator

Demethoxycurcumin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9555 mL | 14.7776 mL | 29.5552 mL | 59.1104 mL | 73.888 mL |
| 5 mM | 0.5911 mL | 2.9555 mL | 5.911 mL | 11.8221 mL | 14.7776 mL |
| 10 mM | 0.2956 mL | 1.4778 mL | 2.9555 mL | 5.911 mL | 7.3888 mL |
| 50 mM | 0.0591 mL | 0.2956 mL | 0.5911 mL | 1.1822 mL | 1.4778 mL |
| 100 mM | 0.0296 mL | 0.1478 mL | 0.2956 mL | 0.5911 mL | 0.7389 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Demethoxycurcumin(Curcumin II) is a major active curcuminoid; possess anti-inflammatory properties; also exert cytotoxic effects in human cancer cells via induction of apoptosis. IC50 value: Target: in vitro: DMC significantly decreased NO secretion by 35-41% in our inflamed cell model. Decrease in NO production by DMC was concomitant with down-regulation of iNOS at mRNA and protein levels compared to proinflammatory cytokine cocktail and LPS-treated controls. Mechanism of action of DMC may be partly due to its potent inhibition of the iNOS pathway [1]. BDMCCN has the strongest inhibitory activity toward BACE-1 with 17 μM IC50, which was 20 and 13 times lower than those of CCN and DMCCN respectively [2]. Genes associated with DNA damage and repair, cell-cycle check point and apoptosis could be altered by DMC; in particular, 144 genes were found up-regulated and 179 genes down-regulated in NCI-H460 cells after exposure to DMC [3]. in vivo: At low doses, both the curcuminoid mixture and curcumin I did not affect brain stimulation reward, whereas, higher doses increased ICSS thresholds. Curcumin II and curcumin III did not affect brain stimulation reward at any doses. Subthreshold doses of the curcuminoid mixture and curcumin I inhibited the reward-facilitating effect of morphine.
References:
[1]. Somchit M, et al. Demethoxycurcumin from Curcuma longa rhizome suppresses iNOS induction in an in vitro inflamed human intestinal mucosa model. Asian Pac J Cancer Prev. 2014;15(4):1807-10.
[2]. Wang X, et al. Effects of curcuminoids identified in rhizomes of Curcuma longa on BACE-1 inhibitory and behavioral activity and lifespan of Alzheimer's disease Drosophila models. BMC Complement Altern Med. 2014 Mar 5;14:88.
[3]. Ko YC, et al. Demethoxycurcumin Alters Gene Expression Associated with DNA Damage, Cell Cycle and Apoptosis in Human Lung Cancer NCI-H460 Cells In Vitro. In Vivo. 2015 01-02;29(1):83-94.
[4]. Katsidoni V, et al. Curcumin, demethoxycurcumin and bisdemethoxycurcumin differentially inhibit morphine's rewarding effect in rats. Psychopharmacology (Berl). 2014 Dec;231(23):4467-78.
- Auriculine
Catalog No.:BCN2013
CAS No.:22595-00-2
- Lucidioline
Catalog No.:BCN7413
CAS No.:22594-91-8
- (+)-Catechin hydrate
Catalog No.:BCN2309
CAS No.:225937-10-0
- Cyclothiazide
Catalog No.:BCC6759
CAS No.:2259-96-3
- Epifriedelanol acetate
Catalog No.:BCN5068
CAS No.:2259-07-6
- PAR 4 (1-6)
Catalog No.:BCC3956
CAS No.:225779-44-2
- Symphytine
Catalog No.:BCN1975
CAS No.:22571-95-5
- Zeorin
Catalog No.:BCN5067
CAS No.:22570-53-2
- Bisabolol Oxide A
Catalog No.:BCC8133
CAS No.:22567-36-8
- Isocryptotanshinone
Catalog No.:BCN2499
CAS No.:22550-15-8
- Robustine
Catalog No.:BCN6653
CAS No.:2255-50-7
- Ocotillone
Catalog No.:BCN5066
CAS No.:22549-21-9
- Kulactone
Catalog No.:BCN7953
CAS No.:22611-36-5
- Methyl kulonate
Catalog No.:BCN7952
CAS No.:22611-37-6
- Cinacalcet
Catalog No.:BCC1483
CAS No.:226256-56-0
- Torachrysone
Catalog No.:BCN5069
CAS No.:22649-04-3
- 4,6,7-Trihydroxycoumarin
Catalog No.:BCC9203
CAS No.:22649-24-7
- Amaronol A
Catalog No.:BCN5070
CAS No.:226560-96-9
- Amaronol B
Catalog No.:BCN5071
CAS No.:226561-02-0
- 3-Hydroxy-12-oleanene-23,28-dioic acid
Catalog No.:BCN1482
CAS No.:226562-47-6
- Fosamprenavir Calcium Salt
Catalog No.:BCC1581
CAS No.:226700-81-8
- Bakkenolide Db
Catalog No.:BCN7117
CAS No.:226711-23-5
- 2-(3,4-dihydroxyphenyl)-2-hydroxypropanoic acid
Catalog No.:BCN6296
CAS No.:22681-72-7
- NPS 2390
Catalog No.:BCC6119
CAS No.:226878-01-9
Anti-Acanthamoebic properties of resveratrol and demethoxycurcumin.[Pubmed:23010569]
Exp Parasitol. 2012 Dec;132(4):519-23.
Acanthamoeba is an opportunist protist pathogen that is known to infect the cornea to produce eye keratitis and the central nervous system to produce fatal granulomatous encephalitis. Early diagnosis, followed by aggressive treatment using a combination of drugs is a prerequisite in successful treatment but even then, prognosis remains poor due to lack of effective drugs. The overall aim of the present study was to determine the anti-Acanthamoebic potential of natural compounds, resveratrol and curcuminoids. Adhesion and cytotoxicity assays were performed using primary human brain microvascular endothelial cells, which constitute the blood-brain barrier. Pre-exposure of organisms to 100 mug resveratrol and demethoxy curcumin prevented amoeba binding by 57% and 73%, respectively, while cytotoxicity of host cells was inhibited by 86%. In an assay for viability of amoebae in the absence of host cells, resveratrol and de-methoxy curcumin exhibited significant amoebicidal effects (23% and 25%, respectively) at 100 mug concentrations (P<0.01). Neither resveratrol nor Demethoxycurcumin had any effect on the proteolytic activities of Acanthamoeba castellanii. Of both compounds, resveratrol is of most interest for further investigation, because of the selective toxicity of resveratrol on A. castellanii but not the human brain microvascular endothelial cells.
DMC is not better than TMZ on intracranial anti-glioma effects.[Pubmed:29575236]
J Cell Biochem. 2018 Jul;119(7):6057-6064.
Previous studies showed Demethoxycurcumin (DMC) has stronger anti-glioma and anti-GSCs effects both in vitro and in vivo. In addition, DMC seems to be lower toxicity than TMZ on nude mice. However, this conclusion was confirmed to be wrong in this study. We have evaluated the antitumor efficacy of DMC or TMZ treatment by an orthotopic glioblastoma xenograft model. Nude mice were injected with U87MG-luc cells in the caudate nucleus of the brain and treated with DMC (30 mg/kg q.d.) or TMZ (10 mg/kg q.d.) by intraperitoneal injection. Bioluminescence imaging (BLI) was used to monitoring tumor growth and response to therapy. Western blot was used to detect the expression of p-Akt, cleaved-caspase-3 and Bax. The average value of BLI showed TMZ determined a significant tumor regression while DMC had a mild regression effect on tumor growth compared with control group. Immunohistochemistry for Ki67, proliferating cell nuclear antigen (PCNA), and TUNEL demonstrated that TMZ more effectively inhibited the expression of Ki67 and PCNA, and increased the ratio of TUNEL-positive cells in in situ tumor tissue. Western blot analysis also indicated that TMZ but not DMC more significantly decreased p-Akt and increased cleaved-caspase-3 and Bax expression.These findings suggested a fact that TMZ appear to be more effective in controlling the growth of glioblastoma than DMC in an orthotopic glioblastoma xenograft model.
Curcumin, demethoxycurcumin and bisdemethoxycurcumin differentially inhibit cancer cell invasion through the down-regulation of MMPs and uPA.[Pubmed:18495463]
J Nutr Biochem. 2009 Feb;20(2):87-95.
Curcumin (Cur), a component of turmeric (Curcuma longa), has been reported to exhibit antimetastatic activities, but the mechanisms remain unclear. Other curcuminoids present in turmeric, Demethoxycurcumin (DMC) and bisDemethoxycurcumin (BDMC) have not been investigated whether they exhibit antimetastatic activity to the same extent as curcumin. The regulation of matrix metalloproteinases (MMPs) and urokinase plasminogen activator (uPA) play important role in cancer cell invasion by cleavage of extracellular matrix (ECM). In this line, we comparatively examined the influence of Cur, DMC and BDMC on the expressions of uPA, MMP-2, MMP-9, membrane Type 1 MMP (MT1-MMP), tissue inhibitor of metalloproteinases (TIMP-2), and in vitro invasiveness of human fibrosarcoma cells. The results indicate that the differential potency for inhibition of cancer cell invasion was BDMC> or =DMC>Cur, whereas the cell migration was not affected. Zymography analysis exhibited that curcumin, DMC and BDMC significantly decreased uPA, active-MMP-2 and MMP-9 but not pro-MMP-2 secretion from the cells in a dose-dependent manner, in which BDMC and DMC show higher potency than curcumin. The suppression of active MMP-2 level correlated with inhibition of MT1-MMP and TIMP-2 protein levels involved in pro-MMP-2 activation. Importantly, BDMC and DMC at 10 microM reduced MT1-MMP and TIMP-2 protein expression, but curcumin slightly reduced only MT1-MMP but not TIMP-2. In addition, three forms of curcuminoids significantly inhibited collagenase, MMP-2, and MMP-9 but not uPA activity. In summary, these data demonstrated that DMC and BDMC show higher antimetastasis potency than curcumin by the differentially down-regulation of ECM degradation enzymes.
Demethoxycurcumin modulates human P-glycoprotein function via uncompetitive inhibition of ATPase hydrolysis activity.[Pubmed:25594233]
J Agric Food Chem. 2015 Jan 28;63(3):847-55.
Curcuminoids are major components of Curcuma longa L., which is widely used as spice in food. This study aimed at identifying whether curcumin, Demethoxycurcumin, and bisDemethoxycurcumin could modulate efflux function of human P-glycoprotein and be used as chemosensitizers in cancer treatments. Without altering P-glycoprotein expression levels and conformation, the purified curcuminoids significantly inhibited P-glycoprotein efflux function. In rhodamine 123 efflux and calcein-AM accumulation assays, Demethoxycurcumin demonstrated the highest inhibition potency (inhibitory IC50 = 1.56 +/- 0.13 muM) among the purified curcuminoids, as well as in the fold of reversal assays. Demethoxycurcumin inhibited P-glycoprotein-mediated ATP hydrolysis under concentrations of <1 muM and efficiently inhibited 200 muM verapamil-stimulated ATPase activity, indicating a high affinity of Demethoxycurcumin for P-glycoprotein. These results suggested that Demethoxycurcumin may be a potential additive natural product in combination with chemotherapeutic agents in drug-resistant cancers.
Demethoxycurcumin, a Natural Derivative of Curcumin Abrogates Rotenone-induced Dopamine Depletion and Motor Deficits by Its Antioxidative and Anti-inflammatory Properties in Parkinsonian Rats.[Pubmed:29576695]
Pharmacogn Mag. 2018 Jan-Mar;14(53):9-16.
Background: Parkinson's disease (PD) is a progressive neurodegenerative disorder (NDD) associated with the loss of dopaminergic neurons in the substantia nigra and subsequently has an effect on motor function and coordination. The pathology of PD is multifactorial, in which neuroinflammation and oxidative damage are the two of the main protagonists. Objectives: The present study aims to assess the potential antioxidant and anti-inflammatory effects of Demethoxycurcumin (DMC), a natural derivative of curcumin, against rotenone-induced PD in rats. Materials and Methods: Rats were randomized and divided into six groups: control, rotenone (0.5 mg/kg/day, intraperitoneal in sunflower oil) treated for 7 days, rotenone and DMC (5, 10, and 20 mg/kg b.w) cotreated, and DMC (20 mg/kg b.w) alone treated groups. Results: Based on the dopamine concentration and biochemical estimations, the effective dose of DMC was selected and the chronic study was performed. At the end of the experimental period, behavioral studies and protein expression patterns of inflammatory markers were analyzed. Rotenone treatment led to motor dysfunctions, neurochemical deficits, and oxidative stress and enhanced expressions of inflammatory markers, whereas oral administration of DMC attenuated all the above. Conclusion: Even though further research is needed to prove its efficacy in clinical trial, the results of our study showed that DMC may offer a promising and new therapeutic lead for the treatment of NDDs including PD. SUMMARY: Curcumin and their derivatives have been shown to be potent neuroprotective effectDemethoxycurcumin (DMC) amolerated the rotenone induced behavioural alterationsDMC abrogated the rotenone induced dopamine deficitsDMC attenuated the rotenone induced oxidative stressDMC diminished the rotenone mediated inflammation. Abbreviations used: COX-2: Cyclooxygenase-2; DA: Dopamine; DMC: Demethoxycurcumin; DMRT: Duncan's multiple range test; GSH: Reduced glutathione; GPx: Glutathione peroxidase; IL-1 beta: Interleukin-1 beta; IL-6: Interleukin-6; iNOS: Inducible nitric oxide synthase; PD: Parkinson's disease; SN: Substantia nigra; SOD: Superoxide dismutase; TBARS: Thiobarbituric acid reactive substances; TNF-alpha: Tumor necrosis factor-alpha.
Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism.[Pubmed:17522064]
Carcinogenesis. 2007 Aug;28(8):1765-73.
Curcumin, a component of turmeric (Curcuma longa), has been shown to exhibit chemopreventive activity. Whether analogs of curcumin (Cur), such as Demethoxycurcumin (DMC), bisDemethoxycurcumin (BDMC), tetrahydrocurcumin (THC) and turmerones, modulate inflammatory signaling and cell proliferation signaling to same extent as curcumin was investigated. The results indicate that the relative potency for suppression of tumor necrosis factor (TNF)-induced nuclear factor-kappaB (NF-kappaB) activation was Cur > DMC > BDMC; thus suggesting the critical role of methoxy groups on the phenyl ring. THC, which lacks the conjugated bonds in the central seven-carbon chain, was completely inactive for suppression of the transcription factor. Turmerones also failed to inhibit TNF-induced NF-kappaB activation. The suppression of NF-kappaB activity correlated with inhibition of NF-kappaB reporter activity and with down-regulation of cyclooxygenase-2, cyclin D1 and vascular endothelial growth factor, all regulated by NF-kappaB. In contrast to NF-kappaB activity, the suppression of proliferation of various tumor cell lines by Cur, DMC and BDMC was found to be comparable; indicating the methoxy groups play minimum role in the growth-modulatory effects of curcumin. THC and turmerones were also found to be active in suppression of cell growth but to a much lesser extent than curcumin, DMC and BDMC. Whether suppression of NF-kappaB or cell proliferation, no relationship of any of the curcuminoid was found with reactive oxygen species (ROS) production. Overall, our results demonstrated that different analogs of curcumin present in turmeric exhibit variable anti-inflammatory and anti-proliferative activities, which do not correlate with their ability to modulate the ROS status.
Demethoxycurcumin modulates prostate cancer cell proliferation via AMPK-induced down-regulation of HSP70 and EGFR.[Pubmed:22849866]
J Agric Food Chem. 2012 Aug 29;60(34):8427-34.
Curcumin (Cur), Demethoxycurcumin (DMC), and bisDemethoxycurcumin (BDMC) are major forms of curcuminoids found in the rhizomes of turmeric. This study examined the effects of three curcuminoid analogues on prostate cancer cells. The results revealed that DMC demonstrated the most efficient cytotoxic effects on prostate cancer PC3 cells. DMC activated AMPK and in turn decreased the activity and/or expression of lipogenic enzymes, such as fatty acid synthase (FASN) and acetyl-CoA carboxylase (ACC). AICAR, an AMPK activator, and DMC down-regulated heat shock protein (HSP) 70 and increased the activity of the pro-apoptotic effector, caspase-3. In addition, DMC sustained epidermal growth factor receptor (EGFR) activation by suppressing the phosphatases PP2a and SHP-2. DMC also increased the interaction between EGFR and Cbl and induced the tyrosine phosphorylation of Cbl. The results suggest that DMC may have antitumor effects on prostate cancer cells via AMPK-induced down-regulation of HSP70 and EGFR.
Demethoxycurcumin inhibits energy metabolic and oncogenic signaling pathways through AMPK activation in triple-negative breast cancer cells.[Pubmed:23777448]
J Agric Food Chem. 2013 Jul 3;61(26):6366-75.
Demethoxycurcumin (DMC), curcumin (Cur), and bisDemethoxycurcumin (BDMC) are major forms of curcuminoids found in the rhizomes of turmeric. This study examined the effects of three curcuminoid analogues on breast cancer cells. The results revealed that DMC demonstrated the most potent cytotoxic effects on breast cancer MDA-MB-231 cells. Compared with estrogen receptor (ER)-positive or HER2-overexpressing breast cancer cells, DMC demonstrated the most efficient cytotoxic effects on triple-negative breast cancer (TNBC) cells. However, nonmalignant MCF-10A cells were unaffected by DMC treatment. The study showed that DMC activated AMPK in TNBC cells. Once activated, AMPK inhibited eukaryotic initiation factor 4E-binding protein-1 (4E-BP1) signaling and mRNA translation via mammalian target of rapamycin (mTOR) and decreased the activity and/or expression of lipogenic enzymes, such as fatty acid synthase (FASN) and acetyl-CoA carboxylase (ACC). DMC also targeted multiple AMPK downstream pathways. Among these, the dephosphorylation of Akt is noteworthy because it circumvents the feedback activation of Akt that results from mTOR inhibition. Moreover, DMC suppressed LPS-induced IL-6 production, thereby blocking subsequent Stat3 activation. In addition, DMC also sustained epidermal growth factor receptor (EGFR) activation by suppressing the phosphatases, PP2a and SHP-2. These results suggest that DMC is a potent AMPK activator that acts through a broad spectrum of anti-TNBC activities.


