Quercetin-3-O-glucuronideCAS# 22688-79-5 |
Quality Control & MSDS
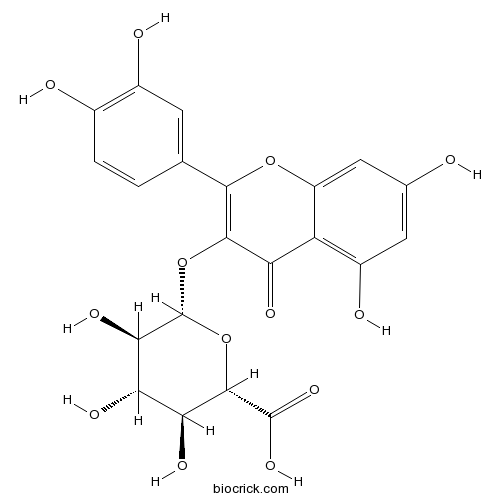
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 22688-79-5 | SDF | Download SDF |
| PubChem ID | 5274585 | Appearance | Yellow powder |
| Formula | C21H18O13 | M.Wt | 478.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Quercetin 3-O-glucuronide; Quercetin 3-glucuronide | ||
| Solubility | DMSO : ≥ 30 mg/mL (62.71 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (2S,3S,4S,5R,6S)-6-[2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxochromen-3-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid | ||
| SMILES | C1=CC(=C(C=C1C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)OC4C(C(C(C(O4)C(=O)O)O)O)O)O)O | ||
| Standard InChIKey | DUBCCGAQYVUYEU-ZUGPOPFOSA-N | ||
| Standard InChI | InChI=1S/C21H18O13/c22-7-4-10(25)12-11(5-7)32-17(6-1-2-8(23)9(24)3-6)18(13(12)26)33-21-16(29)14(27)15(28)19(34-21)20(30)31/h1-5,14-16,19,21-25,27-29H,(H,30,31)/t14-,15-,16+,19-,21+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Quercetin-3-O-glucuronide is a potent stilbene oxidase inhibitor, it has antioxidant, anti-atherogenic, and anti-inflammatory activities. Quercetin-3-O-glucuronide significantly improves Alzheimer's disease (AD)-type deficits in hippocampal formation basal synaptic transmission and long-term potentiation, possibly through mechanisms involving the activation of the c-Jun N-terminal kinases and the mitogen-activated protein kinase signaling pathways. Quercetin-3-O-glucuronide is equally effective in inhibiting ROS-associated inflammation and ameliorating insulin resistant endothelial dysfunction by beneficial regulation of IRS-1 function. |
| Targets | ROS | JNK | MAPK | C/EBPβ | IRS-1 |
| In vitro | Antimicrobial Air Filters Using Natural Euscaphis japonica Nanoparticles.[Pubmed: 25974109]PLoS One. 2015 May 14;10(5):e0126481.Controlling bioaerosols has become more important with increasing participation in indoor activities. Treatments using natural-product nanomaterials are a promising technique because of their relatively low toxicity compared to inorganic nanomaterials such as silver nanoparticles or carbon nanotubes. |
| Kinase Assay | Quercetin represses apolipoprotein B expression by inhibiting the transcriptional activity of C/EBPβ.[Pubmed: 25875015]PLoS One. 2015 Apr 15;10(4):e0121784.Quercetin is one of the most abundant polyphenolic flavonoids found in fruits and vegetables and has anti-oxidative and anti-obesity effects. Because the small intestine is a major absorptive organ of dietary nutrients, it is likely that highly concentrated food constituents, including polyphenols, are present in the small intestinal epithelial cells, suggesting that food factors may have a profound effect in this tissue. |

Quercetin-3-O-glucuronide Dilution Calculator

Quercetin-3-O-glucuronide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0903 mL | 10.4515 mL | 20.903 mL | 41.806 mL | 52.2575 mL |
| 5 mM | 0.4181 mL | 2.0903 mL | 4.1806 mL | 8.3612 mL | 10.4515 mL |
| 10 mM | 0.209 mL | 1.0452 mL | 2.0903 mL | 4.1806 mL | 5.2258 mL |
| 50 mM | 0.0418 mL | 0.209 mL | 0.4181 mL | 0.8361 mL | 1.0452 mL |
| 100 mM | 0.0209 mL | 0.1045 mL | 0.209 mL | 0.4181 mL | 0.5226 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Miquelianin (Quercetin 3-O-glucuronide) is a metabolite of quercetin and a type of natural flavonoid.
In Vitro:Miquelianin shows an antioxidant effect in human plasma. At 50 μM, miquelianin suppresses the consumption of the three antioxidants lycopene, β-carotene and α-tocopherol significantly[1]. In vitro studies indicate that miquelianin is able to reach the central nervous system from the small intestine[2]. Miquelianin significantly reduces the generation of β-amyloid (Aβ) peptides by primary neuron cultures generated from the Tg2576 AD mouse model. It is also capable of interfering with the initial protein-protein interaction of Aβ1–40 and Aβ1–42 that is necessary for the formation of neurotoxic oligomeric Aβ species[3]. Treatment with 0.1 μM miquelianin suppresses ROS generation, cAMP and RAS activation, phosphorylation of ERK1/2 and the expression of HMOX1, MMP2, and MMP9 genes. Miquelianin suppresses invasion of MDA-MB-231 breast cancer cells and MMP-9 induction, and inhibits the binding of [3H]-NA to b2-AR. Miquelianin may function to suppress invasion of breast cancer cells by controlling b2-adrenergic signaling, and may be a dietary chemopreventive factor for stress-related breast cancer[4].
In Vivo:Miquelianin treatment, compared to vehicle-control treatment, significantly improves AD-type deficits in hippocampal formation basal synaptic transmission and long-term potentiation[3]. A flavonoid fraction obtained from a crude extract of Hypericum perforatum (St. John's wort) is remarkably active in the forced swimming test. Miquelianin is one of the compound separated from the fraction[5].
References:
[1]. Terao J, e al. Protection by quercetin and quercetin 3-O-beta-D-glucuronide of peroxynitrite-induced antioxidant consumption in human plasma low-density lipoprotein. Free Radic Res. 2001 Dec;35(6):925-31.
[2]. Juergenliemk G, et al. In vitro studies indicate that miquelianin (quercetin 3-O-beta-D-glucuronopyranoside) is able to reach the CNS from the small intestine. Planta Med. 2003 Nov;69(11):1013-7.
[3]. Butterweck V, et al. Flavonoids from Hypericum perforatum show antidepressant activity in the forced swimming test. Planta Med. 2000 Feb;66(1):3-6.
[4]. Yamazaki S, et al. Quercetin-3-O-glucuronide inhibits noradrenaline-promoted invasion of MDA-MB-231 human breast cancer cells by blocking β₂-adrenergic signaling. Arch Biochem Biophys. 2014 Sep 1;557:18-27.
[5]. Ho L, et al. Identification of brain-targeted bioactive dietary quercetin-3-O-glucuronide as a novel intervention for Alzheimer's disease. FASEB J. 2013 Feb;27(2):769-81.
- Kaempferol-3-beta-O-glucuronide
Catalog No.:BCN2503
CAS No.:22688-78-4
- NPS 2390
Catalog No.:BCC6119
CAS No.:226878-01-9
- 2-(3,4-dihydroxyphenyl)-2-hydroxypropanoic acid
Catalog No.:BCN6296
CAS No.:22681-72-7
- Bakkenolide Db
Catalog No.:BCN7117
CAS No.:226711-23-5
- Fosamprenavir Calcium Salt
Catalog No.:BCC1581
CAS No.:226700-81-8
- 3-Hydroxy-12-oleanene-23,28-dioic acid
Catalog No.:BCN1482
CAS No.:226562-47-6
- Amaronol B
Catalog No.:BCN5071
CAS No.:226561-02-0
- Amaronol A
Catalog No.:BCN5070
CAS No.:226560-96-9
- 4,6,7-Trihydroxycoumarin
Catalog No.:BCC9203
CAS No.:22649-24-7
- Torachrysone
Catalog No.:BCN5069
CAS No.:22649-04-3
- Cinacalcet
Catalog No.:BCC1483
CAS No.:226256-56-0
- Methyl kulonate
Catalog No.:BCN7952
CAS No.:22611-37-6
- Lansoprazole sodium
Catalog No.:BCC4298
CAS No.:226904-00-3
- 4-Hydroxy-1,10-secocadin-5-ene-1,10-dione
Catalog No.:BCN6661
CAS No.:226904-40-1
- LB42708
Catalog No.:BCC5344
CAS No.:226929-39-1
- Emapunil
Catalog No.:BCC5521
CAS No.:226954-04-7
- 3,6-Dimethoxyapigenin
Catalog No.:BCN4830
CAS No.:22697-65-0
- Hyponine E
Catalog No.:BCC8999
CAS No.:226975-99-1
- 9-Deacetyl-9-benzoyl-10-debenzoyl-4beta,20-epoxytaxchinin A
Catalog No.:BCN7676
CAS No.:227011-48-5
- AZ 11645373
Catalog No.:BCC7646
CAS No.:227088-94-0
- (3S,7S)-5,6-Dehydro-4''-de-O-methylcentrolobine
Catalog No.:BCN1481
CAS No.:227289-51-2
- Mucrolidin
Catalog No.:BCN5072
CAS No.:227471-20-7
- Xanthobaccin A
Catalog No.:BCN1864
CAS No.:227596-81-8
- Rubroside G
Catalog No.:BCN1856
CAS No.:227597-42-4
Quercetin represses apolipoprotein B expression by inhibiting the transcriptional activity of C/EBPbeta.[Pubmed:25875015]
PLoS One. 2015 Apr 15;10(4):e0121784.
Quercetin is one of the most abundant polyphenolic flavonoids found in fruits and vegetables and has anti-oxidative and anti-obesity effects. Because the small intestine is a major absorptive organ of dietary nutrients, it is likely that highly concentrated food constituents, including polyphenols, are present in the small intestinal epithelial cells, suggesting that food factors may have a profound effect in this tissue. To identify novel targets of quercetin in the intestinal enterocytes, mRNA profiling using human intestinal epithelial Caco-2 cells was performed. We found that mRNA levels of some apolipoproteins, particularly apolipoprotein B (apoB), are downregulated in the presence of quercetin. On the exposure of Caco-2 cells to quercetin, both mRNA and protein levels of apoB were decreased. Promoter analysis of the human apoB revealed that quercetin response element is localized at the 5'-proximal promoter region, which contains a conserved CCAAT enhancer-binding protein (C/EBP)-response element. We found that quercetin reduces the promoter activity of apoB, driven by the enforced expression of C/EBPbeta. Quercetin had no effect on either mRNA or protein levels of C/EBPbeta. In contrast, we found that quercetin inhibits the transcriptional activity of C/EBPbeta but not its recruitment to the apoB promoter. On the exposure of Caco-2 cells to quercetin 3-O-glucuronide, which is in a cell-impermeable form, no notable change in apoB mRNA was observed, suggesting an intracellular action of quercetin. In vitro interaction experiments using quercetin-conjugated beads revealed that quercetin binds to C/EBPbeta. Our results describe a novel regulatory mechanism of transcription of apolipoprotein genes by quercetin in the intestinal enterocytes.
Antimicrobial Air Filters Using Natural Euscaphis japonica Nanoparticles.[Pubmed:25974109]
PLoS One. 2015 May 14;10(5):e0126481.
Controlling bioaerosols has become more important with increasing participation in indoor activities. Treatments using natural-product nanomaterials are a promising technique because of their relatively low toxicity compared to inorganic nanomaterials such as silver nanoparticles or carbon nanotubes. In this study, antimicrobial filters were fabricated from natural Euscaphis japonica nanoparticles, which were produced by nebulizing E. japonica extract. The coated filters were assessed in terms of pressure drop, antimicrobial activity, filtration efficiency, major chemical components, and cytotoxicity. Pressure drop and antimicrobial activity increased as a function of nanoparticle deposition time (590, 855, and 1150 microg/cm2(filter) at 3-, 6-, and 9-min depositions, respectively). In filter tests, the antimicrobial efficacy was greater against Staphylococcus epidermidis than Micrococcus luteus; ~61, ~73, and ~82% of M. luteus cells were inactivated on filters that had been coated for 3, 6, and 9 min, respectively, while the corresponding values were ~78, ~88, and ~94% with S. epidermidis. Although statistically significant differences in filtration performance were not observed between samples as a function of deposition time, the average filtration efficacy was slightly higher for S. epidermidis aerosols (~97%) than for M. luteus aerosols (~95%). High-performance liquid chromatography (HPLC) and electrospray ionization-tandem mass spectrometry (ESI/MS) analyses confirmed that the major chemical compounds in the E. japonica extract were 1(ss)-O-galloyl pedunculagin, Quercetin-3-O-glucuronide, and kaempferol-3-O-glucoside. In vitro cytotoxicity and disk diffusion tests showed that E. japonica nanoparticles were less toxic and exhibited stronger antimicrobial activity toward some bacterial strains than a reference soluble nickel compound, which is classified as a human carcinogen. This study provides valuable information for the development of a bioaerosol control system that is environmental friendly and suitable for use in indoor environments.


