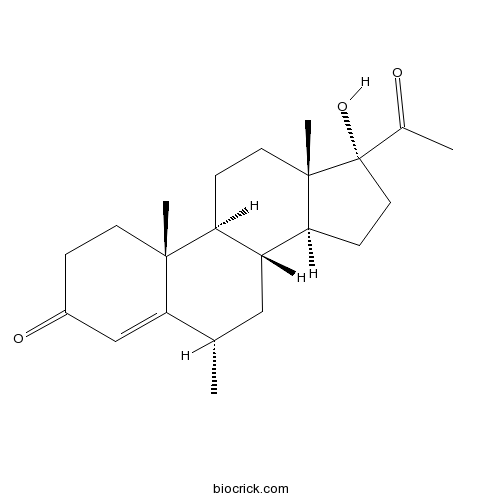
MedroxyprogesteroneCAS# 520-85-4 |
- Salmefamol
Catalog No.:BCC1919
CAS No.:18910-65-1
- Guanfacine hydrochloride
Catalog No.:BCC1609
CAS No.:29110-48-3
- (R,R)-Formoterol
Catalog No.:BCC1293
CAS No.:67346-49-0
- Doxazosin Mesylate
Catalog No.:BCC1257
CAS No.:77883-43-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 520-85-4 | SDF | Download SDF |
| PubChem ID | 10631 | Appearance | Powder |
| Formula | C22H32O3 | M.Wt | 344.49 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 17α-Hydroxy-6α-methylprogesterone; U8840 | ||
| Solubility | DMSO : 10.5 mg/mL (30.48 mM; Need ultrasonic and warming) | ||
| Chemical Name | (6S,8R,9S,10R,13S,14S,17R)-17-acetyl-17-hydroxy-6,10,13-trimethyl-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-3-one | ||
| SMILES | CC1CC2C(CCC3(C2CCC3(C(=O)C)O)C)C4(C1=CC(=O)CC4)C | ||
| Standard InChIKey | FRQMUZJSZHZSGN-HBNHAYAOSA-N | ||
| Standard InChI | InChI=1S/C22H32O3/c1-13-11-16-17(20(3)8-5-15(24)12-19(13)20)6-9-21(4)18(16)7-10-22(21,25)14(2)23/h12-13,16-18,25H,5-11H2,1-4H3/t13-,16+,17-,18-,20+,21-,22-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Medroxyprogesterone Dilution Calculator

Medroxyprogesterone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9028 mL | 14.5142 mL | 29.0284 mL | 58.0568 mL | 72.571 mL |
| 5 mM | 0.5806 mL | 2.9028 mL | 5.8057 mL | 11.6114 mL | 14.5142 mL |
| 10 mM | 0.2903 mL | 1.4514 mL | 2.9028 mL | 5.8057 mL | 7.2571 mL |
| 50 mM | 0.0581 mL | 0.2903 mL | 0.5806 mL | 1.1611 mL | 1.4514 mL |
| 100 mM | 0.029 mL | 0.1451 mL | 0.2903 mL | 0.5806 mL | 0.7257 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Medroxyprogesterone is a progestin, a synthetic variant of the human hormone progesterone and a potent progesterone receptor agonist. Target: Progesterone Receptor Medroxyprogesterone (MP), is a steroidal progestin drug which was never marketed for use in humans. An acylated derivative, medroxyprogesterone acetate (MPA), is clinically used as a pharmaceutical medicine. Compared to MPA, MP is over two orders of magnitude less potent as a progestogen. As such, MP itself is not used clinically, though it has seen limited use in veterinary medicine under the trade name Controlestril in France. In addition, it is an metabolite of MPA [1].
References:
[1]. http://en.wikipedia.org/wiki/Medroxyprogesterone
- Echimidine
Catalog No.:BCN1967
CAS No.:520-68-3
- Rosmarinine
Catalog No.:BCN2124
CAS No.:520-65-0
- Spartioidine
Catalog No.:BCN2134
CAS No.:520-59-2
- Spectabiline
Catalog No.:BCN2098
CAS No.:520-55-8
- Psilocin
Catalog No.:BCC6168
CAS No.:520-53-6
- Asebogenin
Catalog No.:BCN7232
CAS No.:520-42-3
- Apigenin
Catalog No.:BCN5658
CAS No.:520-36-5
- Diosmetin
Catalog No.:BCN2356
CAS No.:520-34-3
- Hesperetin
Catalog No.:BCN5657
CAS No.:520-33-2
- Tricin
Catalog No.:BCN5656
CAS No.:520-32-1
- Tectochrysin
Catalog No.:BCN5655
CAS No.:520-28-5
- Diosimin
Catalog No.:BCN4993
CAS No.:520-27-4
- Isoschaftoside
Catalog No.:BCN3011
CAS No.:52012-29-0
- NCS-382
Catalog No.:BCC6794
CAS No.:520505-01-5
- H-Val-pNA
Catalog No.:BCC3139
CAS No.:52084-13-6
- 2-Hydroxy-7-O-methylscillascillin
Catalog No.:BCN5659
CAS No.:52096-50-1
- Mestanolone
Catalog No.:BCC9022
CAS No.:521-11-9
- Dromostanolone propionate
Catalog No.:BCC8954
CAS No.:521-12-0
- Androstenediol
Catalog No.:BCC8828
CAS No.:521-17-5
- Stanolone
Catalog No.:BCC9153
CAS No.:521-18-6
- Bilobetin
Catalog No.:BCN5661
CAS No.:521-32-4
- Sciadopitysin
Catalog No.:BCN5662
CAS No.:521-34-6
- Cannabinol
Catalog No.:BCN7968
CAS No.:521-35-7
- Pedicin
Catalog No.:BCN4845
CAS No.:521-51-7
Effects of depot-medroxyprogesterone acetate on the immune microenvironment of the human cervix and endometrium: implications for HIV susceptibility.[Pubmed:28051087]
Mucosal Immunol. 2017 Sep;10(5):1270-1278.
Depot-Medroxyprogesterone acetate is a commonly used injectable contraceptive that has been associated with an increased risk of HIV acquisition. This study compares effects of depot-Medroxyprogesterone acetate on immune parameters from several upper reproductive tract compartments relevant to HIV-1 susceptibility in repetitive samples from 15 depot-Medroxyprogesterone acetate users and 27 women not on hormonal contraceptives. Compared with samples from unexposed women in the mid-luteal phase, depot-Medroxyprogesterone acetate use was associated with: increased endocervical concentrations of MCP1 and IFNalpha2; decreased endocervical concentrations of IL1beta and IL6; increased proportions of endometrial CD4+ and CD8+ cells expressing the activation marker HLADR; increased density of endometrial macrophages; and decreased density of endometrial regulatory T cells. Unlike previous reports with samples from the vagina, we did not observe increased expression of the HIV co-receptor CCR5 on CD4+ T cells in the endocervix or endometrium. Our results indicate important differences in anatomic compartments regarding mechanisms by which depot-Medroxyprogesterone acetate could be associated with increased risk of HIV acquisition, including increased recruitment of macrophages to the endometrium, decreased levels of pro-inflammatory cytokines in the endocervix possibly leading to enhanced susceptibility to viral infection, and activation of endometrial T cells.
High dose of green tea infusion normalized spiral artery density in rats treated with the depot-medroxyprogesterone acetate.[Pubmed:28163962]
J Intercult Ethnopharmacol. 2016 Oct 4;6(1):65-67.
AIM: The purpose of this study was to investigate the effects of green tea (GT) on the spiral artery density and endometrial thickness in female rats treated with the depot-Medroxyprogesterone acetate (DMPA). MATERIAL AND METHODS: A total of 24 female rats were randomly divided into four groups (n = 6 each): The control group (no treatment), the DMPA-treated group, treated with DMPA and GT doses of 165 mg/kg of body weight/day, and treated with DMPA and GT doses of 330 mg/kg of body weight/day. Spiral artery density and endometrial thickness were subjected to histopathological analysis. RESULTS: Spiral artery density decreased in the DMPA-treated group, despite the insignificant difference (P > 0.05). With regard to the administration of GT at doses of 165 and 330 mg/g of body weight/day, only GT at the high dose was capable of significantly preventing a decrease in spiral artery density (P < 0.05). At this dose, the spiral arteries achieved a density comparable to that of the control group (P > 0.05). Meanwhile, the administration of DMPA and/or DMPA with GT did not cause significant changes in endometrial thickness relative to the control group (P > 0.05). CONCLUSIONS: DMPA induced a decrease in spiral artery density, despite the insignificant differences, and these changes could be normalized by the administration of high doses of GT. Therefore, GT could be a candidate herb to prevent the adverse effects of the contraceptive DMPA.
Combined Oral Medroxyprogesterone/Levonorgestrel-Intrauterine System Treatment for Women With Grade 2 Stage IA Endometrial Cancer.[Pubmed:28346240]
Int J Gynecol Cancer. 2017 May;27(4):738-742.
OBJECTIVE: The aim of this study was to evaluate the oncologic and pregnancy outcomes of combined oral Medroxyprogesterone acetate (MPA)/levonorgestrel-intrauterine system (LNG-IUS) treatment in young women with grade 2-differentiated stage IA endometrial adenocarcinoma who wish to preserve fertility. METHODS: We retrospectively reviewed the medical records of patients with grade 2 stage IA endometrial adenocarcinoma who had received fertility-sparing treatment at CHA Gangnam Medical Center between 2011 and 2015. All of the patients were treated with combined oral MPA (500 mg/d)/LNG-IUS, and follow-up dilatation and curettage were performed every 3 months. RESULTS: A total of 5 patients were included in the study. The mean age was 30.4 +/- 5.3 years (range, 25-39 years). After a mean treatment duration of 11.0 +/- 6.2 months (range, 6-18 months), complete response (CR) was shown in 3 of the 5 patients, with partial response (PR) in the other 2 patients. One case of recurrence was reported 14 months after achieving CR. This patient was treated again with combined oral MPA/LNG-IUS and achieved CR by 6 months. The average follow-up period was 44.4 +/- 26.2 months (range, 12-71 months). There were no cases of progressive disease. No treatment-related complications arose. CONCLUSIONS: Combined oral MPA/LNG-IUS treatment is considered to be a reasonably effective fertility-sparing treatment of grade 2 stage IA endometrial cancer. Although our results are encouraging, it is preliminary and should be considered with experienced oncologists in well-defined protocol and with close follow-up.
The pregnancy outcome of progestin-primed ovarian stimulation using 4 versus 10 mg of medroxyprogesterone acetate per day in infertile women undergoing in vitro fertilisation: a randomised controlled trial.[Pubmed:28276192]
BJOG. 2017 Jun;124(7):1048-1055.
OBJECTIVE: To investigate the clinical outcome and endocrinological characteristics of progestin-primed ovarian stimulation (PPOS) using 4 versus 10 mg of Medroxyprogesterone acetate (MPA) per day in infertile women with normal ovary reserve. DESIGN: A randomised parallel controlled trial. SETTING: Tertiary-care academic medical centre. PARTICIPANTS: A cohort of 300 infertile women undergoing in vitro fertilisation (IVF)/intracytoplasmic sperm injection (ICSI) treatment. METHODS: Human menopausal gonadotropin (hMG; 225 iu per day) and MPA (group A, 10 mg per day; group B, 4 mg per day) were started simultaneously from cycle day 3 onwards. Ovulation was co-triggered by human chorionic gonadotropin (hCG; 1000 iu) and gonadotropin-releasing hormone agonist (GnRH agonist; 0.1 mg) when dominant follicles matured. Viable embryos were cryopreserved for later frozen embryo transfer (FET) in both groups. MAIN OUTCOME MEASURES: The primary outcome measure was the number of oocytes retrieved. Secondary outcomes included the incidence of a premature surge in luteinising hormone (LH), the number of viable embryos, and clinical pregnancy outcomes. RESULTS: The number of oocytes retrieved and viable embryos were similar between two groups (9.8 +/- 6.3 versus 9.6 +/- 5.9; 4.2 +/- 2.6 versus 3.7 +/- 3.0; P > 0.05). No significant difference was found in clinical pregnancy rate (58.0 versus 48.7%) and live birth rate per participant (48.7 versus 42.0%; P > 0.05). No premature LH surge and ovarian hyperstimulation syndrome (OHSS) occurred in either group. CONCLUSIONS: Progestin-primed ovarian stimulation (PPOS) using 4 or 10 mg of MPA per day was comparable in terms of the number of oocytes retrieved and pregnancy outcome after FET. The administration of 4 mg of MPA per day was sufficient to prevent an untimely LH rise in women undergoing IVF/ICSI treatment. TWEETABLE ABSTRACT: An RCT confirmed similar pregnancy outcome in P-primed ovarian stimulation with a daily dose of 4 or 10 mg MPA.


