trans-MethylisoeugenolCAS# 6379-72-2 |
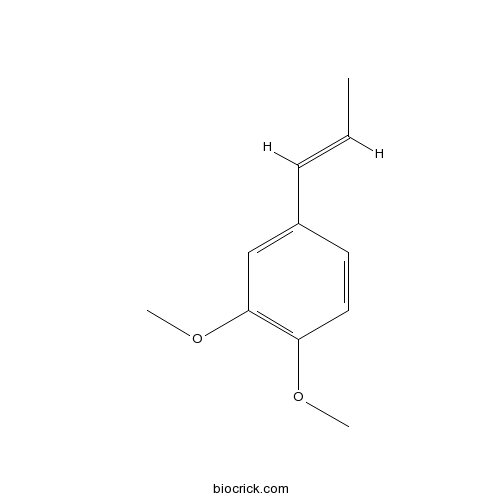
- Methyl isoeugenol
Catalog No.:BCN8462
CAS No.:93-16-3
- cis-Methylisoeugenol
Catalog No.:BCN0620
CAS No.:6380-24-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 6379-72-2 | SDF | Download SDF |
| PubChem ID | 637776 | Appearance | Oil |
| Formula | C11H14O2 | M.Wt | 178.2 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1,2-dimethoxy-4-[(E)-prop-1-enyl]benzene | ||
| SMILES | CC=CC1=CC(=C(C=C1)OC)OC | ||
| Standard InChIKey | NNWHUJCUHAELCL-SNAWJCMRSA-N | ||
| Standard InChI | InChI=1S/C11H14O2/c1-4-5-9-6-7-10(12-2)11(8-9)13-3/h4-8H,1-3H3/b5-4+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Eugenol methyl ether(trans-Methylisoeugenol) can cause moderate reversible inhibition of glutathione S-transferases (GSTs)(I25 ranged from 0.2 to 5.4 mM for human GSTs and from 0.4 to 4.9 mM for rat GSTs). 2.The use of isoeugenol methyl ether(trans-Methylisoeugenol) is described as an agent to inhibit and/or prevent the growth of and/or to destroy micro-organisms causing bad breath and/or to combat bad breath. 3.(E)-Methyl isoeugenol (trans-Methylisoeugenol) is a natural food flavour, it also has anxiolytic and antidepressant like properties. |
| Targets | 5-HT Receptor |

trans-Methylisoeugenol Dilution Calculator

trans-Methylisoeugenol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.6117 mL | 28.0584 mL | 56.1167 mL | 112.2334 mL | 140.2918 mL |
| 5 mM | 1.1223 mL | 5.6117 mL | 11.2233 mL | 22.4467 mL | 28.0584 mL |
| 10 mM | 0.5612 mL | 2.8058 mL | 5.6117 mL | 11.2233 mL | 14.0292 mL |
| 50 mM | 0.1122 mL | 0.5612 mL | 1.1223 mL | 2.2447 mL | 2.8058 mL |
| 100 mM | 0.0561 mL | 0.2806 mL | 0.5612 mL | 1.1223 mL | 1.4029 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cyclosporin D
Catalog No.:BCC6444
CAS No.:63775-96-2
- 12alpha-Hydroxygrandiflorenic acid
Catalog No.:BCN4823
CAS No.:63768-17-2
- 6-Chloromelatonin
Catalog No.:BCC6651
CAS No.:63762-74-3
- 3'-Amino-4'-methoxyacetanilide
Catalog No.:BCC8611
CAS No.:6375-47-9
- AEG 3482
Catalog No.:BCC8088
CAS No.:63735-71-7
- Pseudoprotogracillin
Catalog No.:BCC8354
CAS No.:637349-03-2
- Paspalinine
Catalog No.:BCN7386
CAS No.:63722-91-8
- Z-Phenylalaninol
Catalog No.:BCC2716
CAS No.:6372-14-1
- (R)-Baclofen hydrochloride
Catalog No.:BCC4123
CAS No.:63701-55-3
- 2-(Dimethylamino)ethanesulfonic acid
Catalog No.:BCN1752
CAS No.:637-95-6
- Pramoxine HCl
Catalog No.:BCC4705
CAS No.:637-58-1
- Clofibrate
Catalog No.:BCC5308
CAS No.:637-07-0
- Desonide
Catalog No.:BCC4967
CAS No.:638-94-8
- alpha-Amyrin
Catalog No.:BCN3341
CAS No.:638-95-9
- beta-Amyrone
Catalog No.:BCN4179
CAS No.:638-97-1
- 2-Amino-3-dodecanol
Catalog No.:BCN4175
CAS No.:
- Erythrinin C
Catalog No.:BCN4176
CAS No.:63807-85-2
- Erythrinin A
Catalog No.:BCN3203
CAS No.:63807-86-3
- Dihydroalpinumisoflavone
Catalog No.:BCN4177
CAS No.:63807-90-9
- 5-(3-Chlorophenyl)-N-[4-(morpholin-4-ylmethyl)phenyl]furan-2-carboxamide
Catalog No.:BCC3636
CAS No.:638156-11-3
- Z-D-Arg-OH
Catalog No.:BCC3575
CAS No.:6382-93-0
- Phyllostadimer A
Catalog No.:BCN4178
CAS No.:638203-32-4
- H-Glu-OMe
Catalog No.:BCC2924
CAS No.:6384-08-3
- NMDA (N-Methyl-D-aspartic acid)
Catalog No.:BCC4590
CAS No.:6384-92-5
Anxiolytic and antidepressant like effects of natural food flavour (E)-methyl isoeugenol.[Pubmed:24920211]
Food Funct. 2014 Aug;5(8):1819-28.
(E)-methyl isoeugenol (MIE) is a natural food flavour that constitutes 93.7% of an essential oil from Pimenta pseudocaryophyllus leaf. The leaf extracts of this species are used as a calming agent. As a ubiquitous food additive, the application of MIE for treating mood disorders appears to be globally attractive. Hence, we sought to evaluate general pharmacological activities, anticonvulsant, anxiolytic and antidepressant effects and the possible mechanisms of MIE actions. Administration of MIE was carried out prior to the exposure of a male Swiss mice to general behavioural tests, barbiturate sleep, PTZ-induced convulsion, light dark box (LDB), elevated plus maze (EPM), wire hanging, open field (OF) and forced swimming test (FST). The involvement of monoamine system was studied by mice pretreatment with WAY100635 (antagonist of 5-HT1A), alpha-methyl-p-tyrosine (AMPT; depletor of catecholamine) or p-chlorophenylalanine (PCPA; depletor of serotonin storage). There was no record of neurotoxic effect or animal's death during the course of general pharmacological tests. MIE at 250 and 500 mg kg(-1) potentiated the hypnotic effect of sodium pentobarbital. However, MIE did not protect against PTZ-induced convulsion. Except for MIE at 500 mg kg(-1), parameters evaluated in the LDB, EPM and OF demonstrated an anxiolytic like property of MIE. This effect was blocked by WAY100635 pretreatment. MIE at 500 mg kg(-1) elicited a reduction in locomotor activity of the mice in the OF. Anti-immobility effect of MIE 250 mg kg(-1) in the FST suggested an antidepressive like property. Unlike AMPT, pretreatment with PCPA reversed the antidepressant like effect of MIE. Our findings demonstrated anxiolytic and antidepressant like properties of (E)-methyl isoeugenol and suggested the participation of serotonergic pathways.
Inhibition of rat, mouse, and human glutathione S-transferase by eugenol and its oxidation products.[Pubmed:8620581]
Chem Biol Interact. 1996 Jan 5;99(1-3):85-97.
The irreversible and reversible inhibition of glutathione S-transferases (GSTs) by eugenol was studied in rat, mouse and man. Using liver cytosol of human, rat and mouse, species differences were found in the rate of irreversible inhibition of GSTs by eugenol in the presence of the enzyme tyrosinase. Tyrosinase was used to oxidize eugenol. No inhibition was observed in the absence of tyrosinase. The rate of irreversible inhibition of GSTs was highest in mouse cytosol, and lowest in rat cytosol. In addition, the irreversible inhibition of human and rat GSTs by eugenol was studied using purified isoenzymes of man and rat. The human GST isoenzymes A1-1, M1a-1a and P1-1 and the rat GST isoenzymes 1-1, 2-2, 3-3, 4-4 and 7-7 were irreversibly inhibited by eugenol in the presence of tyrosinase. In this respect human GST P1-1 and rat GST 7-7 were by far the most sensitive enzymes; human GST A2-2 was not inhibited. Indications were found that human GST P1-1 may be inhibited via three mechanisms: in addition to the well documentated nucleophilic addition of quinones and oxidation of cysteine residues, a covalent subunit cross-linking was also observed. The reversible inhibition of human and rat GST by eugenol, eugenol methyl ether, isoeugenol methyl ether, 2-allylphenol and 4-propylphenol was also studied using purified isoenzymes. The reversible inhibition of human and rat GSTs, using 1-chloro-2,4-dinitrobenzene as substrate, was expressed as I25. All compounds caused moderate reversible inhibition (I25 ranged from 0.2 to 5.4 mM for human GSTs and from 0.4 to 4.9 mM for rat GSTs). In rat, eugenol methyl ether was the strongest inhibitor. In human, the overall inhibiting capacities of eugenol, eugenol methyl ether, isoeugenol methyl ether and 4-propyl phenol were more or less similar; 2-allylphenol was the poorest inhibitor.


