(Z)-PugnacInhibitor of O-GlcNAcase and β-hexosaminidase CAS# 132489-69-1 |
- Dihydroberberine
Catalog No.:BCN2573
CAS No.:483-15-8
- Sesamolin
Catalog No.:BCN1289
CAS No.:526-07-8
- Carnosol
Catalog No.:BCN1055
CAS No.:5957-80-2
- Harpagide
Catalog No.:BCN4996
CAS No.:6926-08-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 132489-69-1 | SDF | Download SDF |
| PubChem ID | 9576811 | Appearance | Powder |
| Formula | C15H19N3O7 | M.Wt | 353.33 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
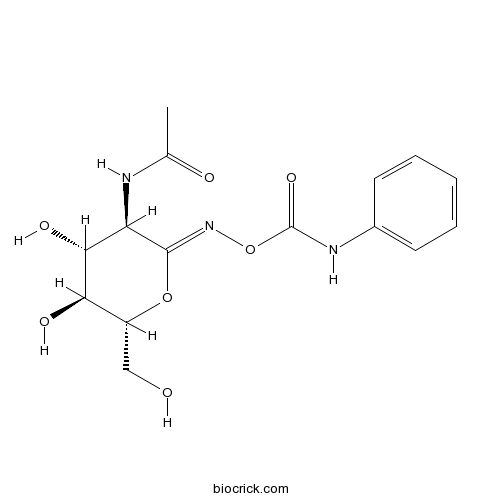
| Chemical Name | [(Z)-[(3R,4R,5S,6R)-3-acetamido-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-ylidene]amino] N-phenylcarbamate | ||
| SMILES | CC(=O)NC1C(C(C(OC1=NOC(=O)NC2=CC=CC=C2)CO)O)O | ||
| Standard InChIKey | PBLNJFVQMUMOJY-JXZOILRNSA-N | ||
| Standard InChI | InChI=1S/C15H19N3O7/c1-8(20)16-11-13(22)12(21)10(7-19)24-14(11)18-25-15(23)17-9-5-3-2-4-6-9/h2-6,10-13,19,21-22H,7H2,1H3,(H,16,20)(H,17,23)/b18-14-/t10-,11-,12-,13-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | O-GlcNAc-β-N-acetylglucosaminidase (O-GlcNAcase) and β-hexosaminidase inhibitor (Ki values are 46 and 36 nM respectively) that increases O-GlcNAc levels ~ 2-fold in HT29 cells. Z-linked isomer is more potent than the E isomer. |

(Z)-Pugnac Dilution Calculator

(Z)-Pugnac Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8302 mL | 14.1511 mL | 28.3022 mL | 56.6043 mL | 70.7554 mL |
| 5 mM | 0.566 mL | 2.8302 mL | 5.6604 mL | 11.3209 mL | 14.1511 mL |
| 10 mM | 0.283 mL | 1.4151 mL | 2.8302 mL | 5.6604 mL | 7.0755 mL |
| 50 mM | 0.0566 mL | 0.283 mL | 0.566 mL | 1.1321 mL | 1.4151 mL |
| 100 mM | 0.0283 mL | 0.1415 mL | 0.283 mL | 0.566 mL | 0.7076 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
(Z)-Pugnac is a potent inhibitor of O-GlcNAcase [1].
Pugnac is an inhibitor of O-GlcNAcase and is initially found to be an inhibitor of β-N-acetylglucosaminidases. It plays its inhibition role through mimicking the substrates of its target enzymes.(Z)-Pugnac is the Z stereoisomer of Pugnac. It is found to be more potent than the E stereoisomer. When tested with the recombinant O-GlcNAcase and the fluorogenic substrate, (Z)-Pugnac inhibited approximately 80% of O-GlcNAcase native activity at concentration of 1 μM. (E)-Pugnac at the same concentration showed only 10% inhibition of the activity. Besides that, (Z)-Pugnac induced about 1.4-fold increased level of O-GlcNAc incorporation on proteins both in HEK cells and in HeLa cells [1].
References:
[1] Perreira M, Kim E J, Thomas C J, et al. Inhibition ofO-GlcNAcase by PUGNAc is dependent upon the oxime stereochemistry. Bioorganic & medicinal chemistry, 2006, 14(3): 837-846.
- 2-TEDC
Catalog No.:BCC6735
CAS No.:132465-10-2
- Alosetron-d3 Hydrochloride
Catalog No.:BCC1345
CAS No.:1189919-71-8
- Neohesperidin
Catalog No.:BCN5915
CAS No.:13241-33-3
- Neoeriocitrin
Catalog No.:BCN3271
CAS No.:13241-32-2
- Chrysophanol 8-O-glucoside
Catalog No.:BCN6175
CAS No.:13241-28-6
- β-D-Glucopyranoside,(3β,6α,16β,20R,24S)-3-[(3,4-di-O-acetyl-β-D-xylopyranosyl)oxy]-20, 24-epoxy-16,25-dihydroxy-9,19-cyclolanostan-6-yl
Catalog No.:BCC8370
CAS No.:1324005-51-7
- TC-G 1006
Catalog No.:BCC6277
CAS No.:1324003-64-6
- Boc-Gln(Trt)-OH
Catalog No.:BCC3384
CAS No.:132388-69-3
- Boc-Asn(Trt)-OH
Catalog No.:BCC3360
CAS No.:132388-68-2
- Fmoc-Gln(Trt)-OPfp
Catalog No.:BCC3486
CAS No.:132388-65-9
- Fmoc-Asn(Trt)-OPfp
Catalog No.:BCC3082
CAS No.:132388-64-8
- Z-Gln(Trt)-OH
Catalog No.:BCC2782
CAS No.:132388-60-4
- Olanzapine
Catalog No.:BCC5042
CAS No.:132539-06-1
- 15-Demethylplumieride
Catalog No.:BCN6176
CAS No.:132586-69-7
- Bruceanic acid C
Catalog No.:BCN7999
CAS No.:132587-60-1
- CCMQ
Catalog No.:BCC6984
CAS No.:132623-44-0
- Otophylloside O
Catalog No.:BCN7337
CAS No.:1326583-08-7
- Senecionine N-oxide
Catalog No.:BCN2130
CAS No.:13268-67-2
- Fmoc-Tle-OH
Catalog No.:BCC2657
CAS No.:132684-60-7
- [Leu31,Pro34]-Neuropeptide Y (human, rat)
Catalog No.:BCC5722
CAS No.:132699-73-1
- PyBrOP
Catalog No.:BCC2821
CAS No.:132705-51-2
- CP 96345
Catalog No.:BCC7509
CAS No.:132746-60-2
- Blonanserin
Catalog No.:BCC3740
CAS No.:132810-10-7
- YS-49
Catalog No.:BCC2067
CAS No.:132836-42-1
Inhibition of O-GlcNAcase by PUGNAc is dependent upon the oxime stereochemistry.[Pubmed:16214344]
Bioorg Med Chem. 2006 Feb 1;14(3):837-46.
The potent O-GlcNAcase inhibitor PUGNAc was synthesized and two isomers based on the E and Z stereochemistry of the oxime moiety were separated, defined, and tested for activity. Several lines of evidence were examined in an effort to define the correct stereochemical assignments of each form of PUGNAc. The ability of the Z stereoisomer to undergo the Beckmann rearrangement was ultimately the most definitive proof. It was determined via both in vitro and intact cell experiments that the Z form of PUGNAc was vastly more potent an inhibitor of O-GlcNAcase than the E form.
O-GlcNAcase uses substrate-assisted catalysis: kinetic analysis and development of highly selective mechanism-inspired inhibitors.[Pubmed:15795231]
J Biol Chem. 2005 Jul 8;280(27):25313-22.
The post-translational modification of serine and threonine residues of nucleocytoplasmic proteins with 2-acetamido-2-deoxy-d-glucopyranose (GlcNAc) is a reversible process implicated in multiple cellular processes. The enzyme O-GlcNAcase catalyzes the cleavage of beta-O-linked GlcNAc (O-GlcNAc) from modified proteins and is a member of the family 84 glycoside hydrolases. The family 20 beta-hexosaminidases bear no apparent sequence similarity yet are functionally related to O-GlcNAcase because both enzymes cleave terminal GlcNAc residues from glycoconjugates. Lysosomal beta-hexosaminidase is known to use substrate-assisted catalysis involving the 2-acetamido group of the substrate; however, the catalytic mechanism of human O-GlcNAcase is unknown. By using a series of 4-methylumbelliferyl 2-deoxy-2-N-fluoroacetyl-beta-D-glucopyranoside substrates, Taft-like linear free energy analyses of these enzymes indicates that O-GlcNAcase uses a catalytic mechanism involving anchimeric assistance. Consistent with this proposal, 1,2-dideoxy-2'-methyl-alpha-D-glucopyranoso-[2,1-d]-Delta2'-thiazoline, an inhibitor that mimics the oxazoline intermediate proposed in the catalytic mechanism of family 20 glycoside hydrolases, is shown to act as a potent competitive inhibitor of both O-GlcNAcase (K(I) = 0.070 microm) and beta-hexosaminidase (K = 0.070 microm). A series of 1,2-dideoxy-2'-methyl-alpha-D-glucopyranoso-[2,1-d]-Delta2'-thiazoline analogues were prepared, and one inhibitor demonstrated a remarkable 1500-fold selectivity for O-GlcNAcase (K(I) = 0.230 microm) over beta-hexosaminidase (K(I) = 340 microm). These inhibitors are cell permeable and modulate the activity of O-GlcNAcase in tissue culture. Because both enzymes have vital roles in organismal health, these potent and selective inhibitors of O-GlcNAcase should prove useful in studying the role of this enzyme at the organismal level without generating a complex chemical phenotype stemming from concomitant inhibition of beta-hexosaminidase.
Purification and characterization of an O-GlcNAc selective N-acetyl-beta-D-glucosaminidase from rat spleen cytosol.[Pubmed:8034696]
J Biol Chem. 1994 Jul 29;269(30):19321-30.
Glycosylation of nuclear and cytoplasmic proteins by O-linked N-acetylglucosamine (O-GlcNAc) monosaccharides is an abundant, ubiquitous, and transient post-translational modification. To characterize enzymes involved in removal of these sugars, a neutral and cytoplasmic N-acetyl-beta-D-glucosaminidase (O-GlcNAcase) with strong selectivity for O-GlcNAc-synthetic glycopeptides has been purified over 22,000-fold from rat spleen homogenate. The purified O-GlcNAcase has two major polypeptides of apparent M(r) = 54,000 (alpha subunit) and M(r) = 51,000 (beta subunit). Enzyme activity sediments at M(r) = 106,000 on sucrose gradients, indicating that the native O-GlcNAcase is an alpha beta heterodimer. The O-GlcNAcase also shows substantially stronger relative activity against O-GlcNAc-synthetic glycopeptides than other hexosaminidases. Unlike acidic lysosomal hexosaminidases, O-GlcNAcase is not inhibited by GalNAc or its analogs, has no other detectable glycosidase activities, and does not cross-react with antibodies against acidic hexosaminidases. Subcellular fractionation and latency studies demonstrate the cytoplasmic and nucleoplasmic localization of the enzyme and its ubiquitous presence in tissues. These studies suggest that O-GlcNAcase is involved in the regulated removal of O-GlcNAc from O-GlcNAc-bearing glycoproteins in the nucleoplasmic and cytoplasmic compartments of cells.


